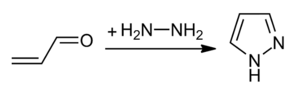Chemistry:Pyrazole
|
| |||
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1H-Pyrazole[1] | |||
| Systematic IUPAC name
1,2-Diazacyclopenta-2,4-diene | |||
| Other names
1,2-Diazole
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C3H4N2 | |||
| Molar mass | 68.079 g·mol−1 | ||
| Melting point | 66 to 70 °C (151 to 158 °F; 339 to 343 K) | ||
| Boiling point | 186 to 188 °C (367 to 370 °F; 459 to 461 K) | ||
| Basicity (pKb) | 11.5 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
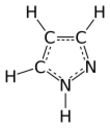
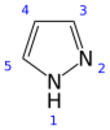
Pyrazole is an organic compound of azole group with the formula C3H3N2H. It is a heterocycle characterized by a 5-membered ring of three carbon atoms and two adjacent nitrogen atoms, which are in ortho-substitution. Pyrazole is a weak base, with pKb 11.5 (pKa of the conjugate acid 2.49 at 25 °C).[2] Pyrazoles are also a class of compounds that have the ring C3N2 with adjacent nitrogen atoms.[3] Notable drugs containing a pyrazole ring are celecoxib (celebrex) and the anabolic steroid stanozolol.
Preparation and reactions
Pyrazoles are synthesized by the reaction of α,β-unsaturated aldehydes with hydrazine and subsequent dehydrogenation:[4]
Substituted pyrazoles are prepared by condensation of 1,3-diketones with hydrazine (Knorr-type reactions).[5] For example, acetylacetone and hydrazine gives 3,5-dimethylpyrazole:[6]
- CH3C(O)CH2C(O)CH3 + N2H4 → (CH3)2C3HN2H + 2 H2O
File:Novel pyrazole ligands.tif
History
The term pyrazole was given to this class of compounds by German Chemist Ludwig Knorr in 1883.[7] In a classical method developed by German chemist Hans von Pechmann in 1898, pyrazole was synthesized from acetylene and diazomethane.[8]
Conversion to scorpionates
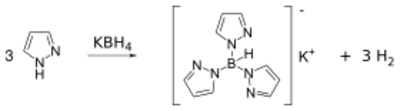
Pyrazoles react with potassium borohydride to form a class of ligands known as scorpionate. Pyrazole itself reacts with potassium borohydride at high temperatures (~200 °C) to form a tridentate ligand known as Tp ligand:
3,5-Diphenyl-1H-pyrazole
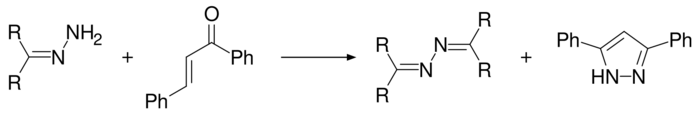
3,5-Diphenyl-1H-pyrazole is produced when (E)-1,3-diphenylprop-2-en-1-one is reacted with hydrazine hydrate in the presence of elemental sulfur[9] or sodium persulfate,[10] or by using a hydrazone in which case an azine is produced as a by-product.[11]
Occurrence and uses

In 1959, the first natural pyrazole, 1-pyrazolyl-alanine, was isolated from seeds of watermelons.[12][13]
In medicine, derivatives of pyrazole are widely used,[14] including celecoxib and similar COX-2 inhibitors, zaleplon, betazole, and CDPPB.[15]
The pyrazole ring is found within a variety of pesticides as fungicides, insecticides and herbicides,[14] including fenpyroximate, fipronil, tebufenpyrad and tolfenpyrad.[16] Pyrazole moieties are listed among the highly used ring systems for small molecule drugs by the US FDA[17]
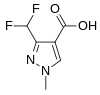
3-(Difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid is used in the manufacture of six commercial fungicides which are inhibitors of succinate dehydrogenase.[18][19]
See also
- 3,5-dimethylpyrazole
- Pyrazolidine, fully saturated analogue
- imidazole, structural analogue of pyrazole with two non-adjacent nitrogen atoms.
- isoxazole, another analogue, the nitrogen atom in position 1 replaced by oxygen.
References
- ↑ "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 141. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ↑ "Dissociation constants of organic acids and bases". http://sites.chem.colostate.edu/diverdi/all_courses/CRC%20reference%20data/dissociation%20constants%20of%20organic%20acids%20and%20bases.pdf.
- ↑ Eicher, T.; Hauptmann, S. (2003). The Chemistry of Heterocycles: Structure, Reactions, Syntheses, and Applications (2nd ed.). Wiley-VCH. ISBN 3-527-30720-6.
- ↑ Schmidt, Andreas; Dreger, Andrij (2011). "Recent Advances in the Chemistry of Pyrazoles. Properties, Biological Activities, and Syntheses". Curr. Org. Chem. 15 (9): 1423–1463. doi:10.2174/138527211795378263.
- ↑ Nozari, M., Addison, A., Reeves, G.T, Zeller, M., Jasinski, J.P., Kaur, M., Gilbert, J. G., Hamilton, C. R., Popovitch, J. M., Wolf, L. M., Crist, L. E., Bastida, N., (2018) Journal of heterocyclic Chemistry 55, 6, 1291-1307. https://doi.org/10.1002/jhet.3155.
- ↑ Johnson, William S.; Highet, Robert J. (1963). "3,5-Dimethylpyrazole". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=CV4P0351.; Collective Volume, 4, pp. 351
- ↑ Knorr, L. (1883). "Action of ethyl acetoacetate on phenylhydrazine. I". Chemische Berichte 16: 2597–2599. doi:10.1002/cber.188301602194. https://zenodo.org/record/1425305.
- ↑ von Pechmann, Hans (1898). "Pyrazol aus Acetylen und Diazomethan" (in German). Berichte der deutschen chemischen Gesellschaft 31 (3): 2950–2951. doi:10.1002/cber.18980310363. https://zenodo.org/record/1425922.
- ↑ Outirite, Moha; Lebrini, Mounim; Lagrenée, Michel; Bentiss, Fouad (2008). "New one step synthesis of 3,5-disubstituted pyrazoles under microwave irradiation and classical heating". Journal of Heterocyclic Chemistry 45 (2): 503–505. doi:10.1002/jhet.5570450231.
- ↑ Zhang, Ze; Tan, Ya-Jun; Wang, Chun-Shan; Wu, Hao-Hao (2014). "One-pot synthesis of 3,5-diphenyl-1H-pyrazoles from chalcones and hydrazine under mechanochemical ball milling". Heterocycles 89 (1): 103–112. doi:10.3987/COM-13-12867.
- ↑ Lasri, Jamal; Ismail, Ali I. (2018). "Metal-free and FeCl3-catalyzed synthesis of azines and 3,5-diphenyl-1H-pyrazole from hydrazones and/or ketones monitored by high resolution ESI+-MS". Indian Journal of Chemistry, Section B 57B (3): 362–373. http://nopr.niscair.res.in/handle/123456789/43824.
- ↑ Fowden; Noe; Ridd; White (1959). Proc. Chem. Soc.: 131.
- ↑ Noe, F. F.; Fowden, L.; Richmond, P. T. (1959). "alpha-Amino-beta-(pyrazolyl-N) propionic acid: a new amino-acid from Citrullus vulgaris (water melon)". Nature 184 (4688): 69–70. doi:10.1038/184069a0. PMID 13804343. Bibcode: 1959Natur.184...69B.
- ↑ 14.0 14.1 Kabi, Arup K.; Sravani, Sattu; Gujjarappa, Raghuram et al. (2022). "Overview on Biological Activities of Pyrazole Derivatives". Nanostructured Biomaterials. Materials Horizons: From Nature to Nanomaterials. pp. 229–306. doi:10.1007/978-981-16-8399-2_7. ISBN 978-981-16-8398-5.
- ↑ Faria, Jéssica Venância; Vegi, Percilene Fazolin; Miguita, Ana Gabriella Carvalho; dos Santos, Maurício Silva; Boechat, Nubia; Bernardino, Alice Maria Rolim (1 November 2017). "Recently reported biological activities of pyrazole compounds". Bioorganic & Medicinal Chemistry 25 (21): 5891–5903. doi:10.1016/j.bmc.2017.09.035. ISSN 0968-0896. PMID 28988624.
- ↑ FAO
- ↑ Taylor, R. D.; MacCoss, M.; Lawson, A. D. G. J Med Chem 2014, 57, 5845.
- ↑ Walter, Harald (2016). "Fungicidal Succinate-Dehydrogenase-Inhibiting Carboxamides". Bioactive Carboxylic Compound Classes: Pharmaceuticals and Agrochemicals. Wiley. pp. 405–425. doi:10.1002/9783527693931.ch31. ISBN 9783527339471.
- ↑ Jeschke, Peter (2021). "Current Trends in the Design of Fluorine‐Containing Agrochemicals". Organofluorine Chemistry. Wiley. pp. 363–395. doi:10.1002/9783527825158.ch11. ISBN 9783527347117.
Further reading
A. Schmidt; A. Dreger (2011). "Recent Advances in the Chemistry of Pyrazoles. Part 2. Reactions and N-Heterocyclic Carbenes of Pyrazole". Curr. Org. Chem. 15 (16): 2897–2970. doi:10.2174/138527211796378497.
 |