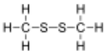Chemistry:Dimethyl disulfide
|
| |||
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
(Methyldisulfanyl)methane[1] | |||
| Other names
Dimethyl disulfide[1]
Methyl disulfide Methyldisulfide Dimethyldisulfide Methyldithiomethane 2,3-Dithiabutane | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| Abbreviations | DMDS | ||
| ChEBI | |||
| ChemSpider | |||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| CH 3SSCH 3 | |||
| Molar mass | 94.19 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Density | 1.06 g/cm3[2] | ||
| Melting point | −85 °C (−121 °F; 188 K)[2] | ||
| Boiling point | 110 °C (230 °F; 383 K)[2] | ||
| 2.5 g/L (20 °C)[2] | |||
| Vapor pressure | 3.8 kPa (at 25 °C) Arkema data sheet | ||
| Hazards | |||
| Flash point | 15 °C (59 °F; 288 K)[2] | ||
| 370 °C (698 °F; 643 K)[2] | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
190 mg/kg (oral, rat)[3] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Dimethyl disulfide (DMDS) is an organic chemical compound with the molecular formula CH
3SSCH
3. It is a flammable liquid with an unpleasant, garlic-like odor. The compound is colorless although impure samples often appear yellowish.
Occurrence and synthesis
Dimethyl disulfide is widespread in nature. It is emitted by bacteria, fungi, plants, and animals. Along with dimethyl sulfide and dimethyl trisulfide it has been confirmed as volatile compounds given off by the fly-attracting plant known as dead-horse arum (Helicodiceros muscivorus). These flies are attracted to the odor resembling that of fetid meat, and thus help pollinate this plant.[4]
DMDS can be produced by the oxidation of methanethiol, e.g. with iodine:
- 2 CH
3SH + I
2 → CH
3SSCH
3 + 2 HI
Chemical reactions
Important reactions include chlorination giving methanesulfenyl chloride (CH
3SCl), methanesulfinyl chloride (CH
3S(O)Cl),[5] and methanesulfonyl chloride (CH
3SO
2Cl) as well as oxidation with hydrogen peroxide or peracetic acid giving the thiosulfinate methyl methanethiosulfinate (CH
3S(O)SCH
3).[6]
Uses
DMDS is used as a food additive in onion, garlic, cheese, meats, soups, savory flavors, and fruit flavors.[7] Industrially, DMDS is used in oil refineries as a sulfiding agent.[8] DMDS is also an effective soil fumigant in agriculture, registered in many states in the U.S. as well as globally. In this capacity, DMDS is an important alternative in replacing methyl bromide, which is being phased out, however less effective than the former. This pesticide is marketed as "Paladin" by Arkema.[9][10]
Industrial use
DMDS is used to prepare catalysts for hydrodesulfurization, because of its high sulfur content and low decomposition temperature. Refineries utilize DMDS instead of other sulfur spiking agents for catalyst sulfiding because it has more sulfur per pound than dimethyl sulfide (DMS) or di-tertiary-butyl polysulfide (TBPS).[11] Once injected to a hydrotreater or hydrocracker, DMDS decomposes to form H2S. The H2S reacts with the metal oxides on the catalyst, converting them to the active metal sulfide form.[12]
DMDS also works as an effective product for operators in the petrochemicals industry who must protect their steam-cracking coils against the formation of coke and carbon monoxide.
DMDS is utilized in the preparation of 4-(methylthio)phenol which is used in the production of various pesticides. DMDS and chlorine are reacted with borontrifluoride phenoxide to produce 4-(methylthio)phenol. Thiophene and DMDS are blended with combustible hydrocarbon fuel gas to impart a gassy odor to the fuel gas.
References
- ↑ 1.0 1.1 Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 708. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ↑ [1], EPA DMDS Fact Sheet
- ↑ Marcus C. Stensmyr; Isabella Urru; Ignazio Collu; Malin Celander; Bill S. Hansson; Anna-Maria Angioy (2002). "Rotting smell of dead-horse arum florets". Nature 420 (6916): 625–626. doi:10.1038/420625a. PMID 12478279.
- ↑ Irwin B. Douglass and Richard V. Norton "Methanesulfinyl Chloride" Organic Syntheses, Coll. Vol. 5, p.709-712 (1973).
- ↑ Block, Eric; O'Connor, John (1974). "Chemistry of Alkyl Thiosulfinate Esters. VI. Preparation and Spectral Studies". Journal of the American Chemical Society 96 (12): 3921. doi:10.1021/ja00819a033.
- ↑ [2], OSHA
- ↑ Dimethyl Disulfide (DMDS) , Arkema, Inc.
- ↑ "DMDS for agricultural soil fumigation". Arkema. http://www.arkema.com/en/products/product-finder/range-viewer/DMDS-for-agricultural-soil-fumigation/. Retrieved 2013-09-06.
- ↑ Registration of Paladin and Paladin EC containing the new active ingredient dimethyl disulfide. New York State Department of Environmental Conservation. March 9, 2012. http://pmep.cce.cornell.edu/profiles/fumigant/dimethyl_disulfide/dimethyl_disulfide_reg_0312.pdf.
- ↑ Dimethyl Disulfide (DMDS)
- ↑ "Reactor Resources". http://www.reactor-resources.com/sulfiding-services/sulfiding-101.html.
 |