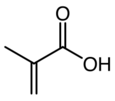
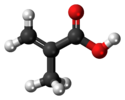
Chemistry:Methacrylic acid
|
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Methacrylic acid[1]
| |||
| Preferred IUPAC name
2-Methylprop-2-enoic acid | |||
| Other names
Methacrylic acid
2-Methyl-2-propenoic acid α-Methacrylic acid 2-Methylacrylic acid 2-Methylpropenoic acid | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| Abbreviations | MAA | ||
| ChEBI | |||
| ChemSpider | |||
| EC Number |
| ||
| MeSH | C008384 | ||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C4H6O2 | |||
| Molar mass | 86.09 g/mol | ||
| Appearance | Colorless liquid | ||
| Odor | Acrid, repulsive[2] | ||
| Density | 1.015 g/cm3 | ||
| Melting point | 14 to 15 °C (57 to 59 °F; 287 to 288 K) | ||
| Boiling point | 161 °C (322 °F; 434 K) | ||
| 9% (25 °C)[2] | |||
| Vapor pressure | 0.7 mmHg (20 °C)[2] | ||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 77.2 °C (171.0 °F; 350.3 K) | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
none[2] | ||
REL (Recommended)
|
TWA 20 ppm (70 mg/m3) [skin][2] | ||
IDLH (Immediate danger)
|
N.D.[2] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Methacrylic acid, abbreviated MAA, is an organic compound with the formula CH2=C(CH3)COOH. This colorless, viscous liquid is a carboxylic acid with an acrid unpleasant odor. It is soluble in warm water and miscible with most organic solvents. Methacrylic acid is produced industrially on a large scale as a precursor to its esters, especially methyl methacrylate (MMA), and to poly(methyl methacrylate) (PMMA).
Production
In the most common route, methacrylic acid is prepared from acetone cyanohydrin, which is converted to methacrylamide sulfate using sulfuric acid. This derivative in turn is hydrolyzed to methacrylic acid, or esterified to methyl methacrylate in one step. Another route to methacrylic acid starts with isobutylene, which obtainable by dehydration of tert-butanol. Isobutylene is oxidized sequentially to methacrolein and then methacrylic acid. Methacrolein for this purpose can also be obtained from formaldehyde and ethylene. Yet a third route involves the dehydrogenation of Isobutyric acid.[3]
Various green routes have been explored but they have not been commercialized. Specifically, the decarboxylation of itaconic acid, citraconic acid, and mesaconic acids affords methacrylic acid.[4] Salts of methacrylic acid have been obtained by boiling citra- or meso-brompyrotartaric acids with alkalis.[citation needed]
Pyrolysis of ethyl methacrylate efficiently gives methacrylic acid.[5]
Uses and occurrence
The main use of methacrylic acid is its polymerization to poly(methyl methacrylate).[6]
It is used in some nail primers to help acrylic nails adhere to the nail plate.[7]
Copolymers consisting partially of methacrylic acid are used in certain types of tablet coatings in order to slow the tablet's dissolution in the digestive tract, and thus extend or delay the release of the active ingredient.[8]
MAA occurs naturally in small amounts in the oil of Roman chamomile.[citation needed]
Reactions
For a commercial applications, MMA is polymerized using azobisisobutyronitrile as a thermally activated free-radical catalyst. Otherwise, MMA is relatively slow to polymerize thermally or photochemically.[6]
Methacrylic acid undergoes several reactions characteristic of alpha,beta-unsaturated acids (see acrylic acid). These reactions include the Diels–Alder reaction and Michael additions. Esterifications are brought about by acid-catalyzed condensations with alcohols, alkylations with certain alkenes, and transesterifications. Epoxide ring-opening gives hydroxyalkyl esters.[3] Sodium amalgam reduces it to isobutyric acid. A polymeric form of methacrylic acid was described in 1880.[9]
See also
thumb|352px|left|Typical [[vinyl ester resin derived from bisphenol A diglycidyl ether and methacrylic acid.[10]]]
References
- ↑ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 746. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 NIOSH Pocket Guide to Chemical Hazards. "#0386". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0386.html.
- ↑ 3.0 3.1 William Bauer Jr. (2002). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a16_441.
- ↑ Le Nôtre, Jérôme; Witte-van Dijk, Susan C. M.; van Haveren, Jacco; Scott, Elinor L.; Sanders, Johan P. M. (September 2014). "Synthesis of Bio-Based Methacrylic Acid by Decarboxylation of Itaconic Acid and Citric Acid Catalyzed by Solid Transition-Metal Catalysts" (in en). ChemSusChem 7 (9): 2712–2720. doi:10.1002/cssc.201402117. PMID 25045161. https://onlinelibrary.wiley.com/doi/10.1002/cssc.201402117.
- ↑ W. P. Ratchford (1949). "Acrylic Acid I. Pyrolysis Method". Organic Syntheses 29: 2. doi:10.15227/orgsyn.029.0002. This article also describes pyrolysis of ethyl methacrylate.
- ↑ 6.0 6.1 Stickler, Manfred; Rhein, Thoma (2000). "Polymethacrylates". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a21_473. ISBN 3-527-30673-0.
- ↑ "Products - Nail Care Products" (in en). U.S. Food and Drug Administration. 2018-03-06. https://www.fda.gov/cosmetics/productsingredients/products/ucm127068.htm#mono.
- ↑ "Aqueous enteric coatings with methacrylic acid copolymer type C on acidic and basic drugs in tablets and pellets, part I: Acetylsalicylic acid tablets and crystals". https://www.researchgate.net/publication/287485833.
- ↑ F. Engelhorn (1880). Rudolph Fittig. ed. "II. Untersuchungen über die ungesättigten Säuren. Zur Kenntnis der Methacrylsäure". Justus Liebigs Annalen der Chemie 200: 70. doi:10.1002/jlac.18802000103. https://zenodo.org/record/1655491..
- ↑ Pham, Ha Q.; Marks, Maurice J. (2012). "Epoxy Resins". Ullmann's Encyclopedia of Industrial Chemistry (Weinheim: Wiley-VCH). doi:10.1002/14356007.a09_547.pub2. ISBN 978-3-527-30673-2.
 |