Chemistry:Aluminium cyanide
From HandWiki
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C3AlN3 | |
| Molar mass | 105.036 g·mol−1 |
| Appearance | white solid |
| Reacts | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
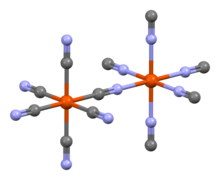
Aluminium cyanide is a metallic cyanide with a chemical formula of Al(CN)3.[1] It is a white solid that undergoes hydrolysis to produce aluminium hydroxide and hydrogen cyanide.[2]
Synthesis and properties
Aluminium cyanide was first produced in 1924 as its ammoniate, Al(CN)3·5NH3, by reacting aluminium metal and mercury(II) cyanide in liquid ammonia to prevent hydrolysis.[1]
- 2 Al + 3 Hg(CN)2 → 2 Al(CN)3 + 3 Hg
When the ammoniate contacts water, it produces aluminium hydroxide, ammonia, and ammonium cyanide.[1]
The pure compound was produced in 2001 by the reaction of lithium tetrachloroaluminate and trimethylsilyl cyanide in diethyl ether and its crystals form an octahedral Prussian-blue-type structure.[3]
References
- ↑ 1.0 1.1 1.2 Bergstrom, F. W. (July 1924). "The Reaction Between Mercuric Cyanide and Certain Metals in Liquid Ammonia". Journal of the American Chemical Society 46 (7): 1559–1568. doi:10.1021/ja01672a002.
- ↑ Axel Schulz; Jonas Surkau (2022). "Main group cyanides: from hydrogen cyanide to cyanido-complexes" (in en). Reviews in Inorganic Chemistry 43 (1): 49–188. doi:10.1515/revic-2021-0044.
- ↑ Darrick Williams; Brett Pleune; Kurt Leinenweber; J. Kouvetakis (2001). "Synthesis and Structural Properties of the Binary Framework C–N Compounds of Be, Mg, Al, and Tl" (in en). Journal of Solid State Chemistry 159 (1): 244–250. doi:10.1006/jssc.2001.9192. Bibcode: 2001JSSCh.159..244W.
 |

