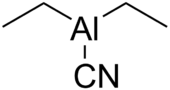
Chemistry:Diethylaluminium cyanide
| |
| Names | |
|---|---|
| IUPAC name
diethylalumanylformonitrile
| |
Other names
| |
| Identifiers | |
PubChem CID
|
|
| Properties | |
| (CH 3CH 2) 2AlCN | |
| Molar mass | 111.124 g·mol−1 |
| Appearance | Dark brown, clear liquid (1.0 mol/L in toluene)[1] |
| Density | 0.864 g/cm3 at (25 °C) (liquid) |
| Boiling point | 162 °C (324 °F; 435 K) at 0.02 mmHg |
| Reacts with water | |
| Solubility | Benzene, Toluene, diisopropyl ether |
| Hazards | |
| Flash point | 7 °C (45 °F; 280 K) closed cup[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Diethylaluminium cyanide ("Nagata's reagent")[2] is the organoaluminium compound with formula ((C
2H
5)
2AlCN)
n. This colorless compound is usually handled as a solution in toluene. It is a reagent for the hydrocyanation of α,β-unsaturated ketones.[1][3][4][5][6]
Synthesis
Diethylaluminium cyanide was originally generated by treatment of triethylaluminium with a slight excess of hydrogen cyanide. The product is typically stored in ampoules because it is highly toxic. It dissolves in toluene, benzene, hexane and isopropyl ether. It undergoes hydrolysis readily and is not compatible with protic solvents.
- n Et
3Al + n HCN → (Et
2AlCN)
n + n EtH
Structure
Diethylaluminium cyanide has not been examined by X-ray crystallography, although other diorganoaluminium cyanides have been. Diorganylaluminium cyanides have the general formula (R
2AlCN)
n, and they exist as cyclic trimers (n = 3) or tetramers (n = 4). In these oligomers, one finds AlCN---Al linkages. One compound similar to diethylaluminium cyanide is bis[di(trimethylsilyl)methyl]aluminium cyanide, ((Me
3Si)
2CH)
2AlCN, which has been shown crystallographically to exist as a trimer with the following structure:[4]
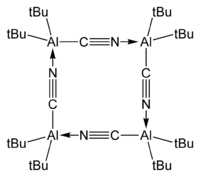
Bis(tert-butyl)aluminium cyanide, t
Bu
2AlCN exists as a tetramer in the crystalline phase:[7][8]
Uses
Diethylaluminium cyanide is used for the stoichiometric hydrocyanation of α,β-unsaturated ketones. The reaction is influenced by the basicity of the solvent. This effect arises from the Lewis acidic qualities of the reagent.[9] The purpose of this reaction is to generate alkylnitriles, which are precursors to amines, amides, carboxylic acids esters and aldehydes.
References
- ↑ Jump up to: 1.0 1.1 1.2 "MSDS - 276863". Sigma-Aldrich. http://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=AU&language=en&productNumber=276863&brand=ALDRICH&PageToGoToURL=http%3A%2F%2Fwww.sigmaaldrich.com%2Fcatalog%2Fproduct%2Faldrich%2F276863%3Flang%3Den.
- ↑ Nagata, W (1988). "Diethylaluminum cyanide". Organic Syntheses VI: 307. doi:10.15227/orgsyn.052.0090.
- ↑ Nagata, W. (1966). "Alkylaluminum cyanides as potent reagents for hydrocyanation". Tetrahedron Lett. 7 (18): 1913–1918. doi:10.1016/S0040-4039(00)76271-X.
- ↑ Jump up to: 4.0 4.1 Uhl, Werner; Schütz, Uwe; Hiller, Wolfgang; Heckel, Maximilian (1995). "Synthese und Kristallstruktur des trimeren [(Me3Si)2CH]2Al—CN". Z. anorg. allg. Chem. 621 (5): 823–828. doi:10.1002/zaac.19956210521.
- ↑ Wade, K.; Wyatt, B. K. (1969). "Reactions of organoaluminium compounds with cyanides. Part III. Reactions of trimethylaluminium, triethylaluminium, dimethylaluminium hydride, and diethylaluminium hydride with dimethylcyanamide". J. Chem. Soc.: 1121–1124. doi:10.1039/J19690001121.
- ↑ Coates, G. E.; Mukherjee, R. N. (1963). "35. Dimethylaluminium cyanide and its gallium, indium, and thallium analogues; beryllium and methylberyllium cyanide". J. Chem. Soc.: 229–232. doi:10.1039/JR9630000229.
- ↑ Uhl, W.; Matar, M. (2004). "Hydroalumination of nitriles and isonitriles". Z. Naturforsch. B 59 (11–12): 1214–1222. doi:10.1515/znb-2004-11-1239. http://www.znaturforsch.com/ab/v59b/59b1214.pdf.
- ↑ Uhl, W.; Schütz, U.; Hiller, W.; Heckel, M. (2005). "Synthese und Kristallstruktur des trimeren [(Me3Si)2CH]2Al—CN". Z. Naturforsch. B 60 (2): 155–163. http://www.znaturforsch.com/ab/v60b/60b0155.pdf.
- ↑ Nagata, W.; Yoshioka, M. (1988). "Diethylaluminum cyanide". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=cv6p0436.; Collective Volume, 6, pp. 436
External links
 |
![trimeric bis[di(trimethylsilyl)methyl]aluminium cyanide](/wiki/images/thumb/8/8f/Cyclo-%28%28%28Me3Si%292CH%292AlCN%293-2D.png/300px-Cyclo-%28%28%28Me3Si%292CH%292AlCN%293-2D.png)