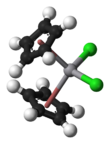
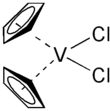Chemistry:Vanadocene dichloride
|
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Dichlorobis(η5-cyclopentadienyl) vanadium
| |||
| Other names
Dicyclopentadienyl vanadium dichloride
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| Abbreviations | Cp2VCl2 | ||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 3285 | ||
| |||
| Properties | |||
| C10H10Cl2V | |||
| Molar mass | 252.03 g/mol | ||
| Appearance | Green solid | ||
| Density | 1.7 g/ml | ||
| Melting point | decomposes | ||
| Boiling point | decomposes | ||
| Soluble (Hydrolysis) | |||
| Structure | |||
| Monoclinic | |||
| Tetrahedral | |||
| Hazards | |||
| Main hazards | Irritant | ||
| GHS pictograms |  
| ||
| GHS Signal word | Danger | ||
| H301, H315, H319, H335 | |||
| P261, P264, P270, P271, P280, P301+310, P302+352, P304+340, P305+351+338, P312, P321, P330, P332+313, P337+313, P362, P403+233, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Related compounds | |||
Related compounds
|
Titanocene dichloride Zirconocene dichloride Hafnocene dichloride Niobocene dichloride Tantalocene dichloride Molybdenocene dichloride Tungstenocene dichloride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Vanadocene dichloride is an organometallic complex with formula (η5-C5H5)2VCl2 (commonly abbreviated as Cp2VCl2). It is a structural analogue of titanocene dichloride but with vanadium(IV) instead of titanium(IV). This compound has one unpaired electron, hence Cp2VCl2 is paramagnetic. Vanadocene dichloride is a suitable precursor for variety of bis(cyclopentadienyl)vanadium(IV) compounds.
Preparation
Cp2VCl2 was first prepared by Wilkinson and Birmingham via the reaction of NaC5H5 and VCl4 in THF.[1]
Reactions and use
The compound has been used in organic synthesis.[2]
Reduction of vanadocene dichloride gives vanadocene, (C5H5)2V.
Like titanocene dichloride, this organovanadium compound was investigated as a potential anticancer drug. It was conjectured to function by interactions with the protein transferrin.[3]
References
- ↑ Wilkinson, G.; Birmingham, J. G. (1954). "Bis-cyclopentadienyl Compounds of Ti, Zr, V, Nb and Ta". J. Am. Chem. Soc. 76 (17): 4281–4284. doi:10.1021/ja01646a008.
- ↑ Hirao, T.; Ogawa, A.; Asahara, M.; Muguruma, Y.; Sakurai, H. (2005). "d,l-Selective Pinacol-Type Coupling Using Zinc, Chlorosilane, and Catalytic Amount of Cp2VCl2; dl-1,2-Dicyclohexylethanediol". Organic Syntheses 81: 26. http://www.orgsyn.org/demo.aspx?prep=v81p0026.
- ↑ Honzíček, Jan; Vinklárek, Jaromír (2015). "Bioinorganic chemistry of vanadocene dichloride". Inorganica Chimica Acta 437: 87–94. doi:10.1016/j.ica.2015.08.008.
 |