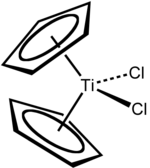
Chemistry:Titanocene dichloride
| |
| |

| |
| Names | |
|---|---|
| IUPAC name
Dichloridobis(η5-cyclopentadienyl)titanium
| |
| Other names
titanocene dichloride, dichlorobis(cyclopentadienyl)titanium(IV)
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 3261 |
| |
| |
| Properties | |
| C10H10Cl2Ti | |
| Molar mass | 248.96 g/mol |
| Appearance | bright red solid |
| Density | 1.60 g/cm3, solid |
| Melting point | 289 °C (552 °F; 562 K) |
| sl. sol. with hydrolysis | |
| Structure | |
| Triclinic | |
| Dist. tetrahedral | |
| Hazards[1] | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H335 | |
| P201, P202, P261, P264, P270, P271, P280, P281, P301+310, P301+312, P302+352, P304+340, P305+351+338, P308+313, P312, P330, P332+313, P337+313, P362, P403+233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Related compounds
|
Ferrocene Zirconocene dichloride Hafnocene dichloride Vanadocene dichloride Niobocene dichloride Tantalocene dichloride Molybdocene dichloride Tungstenocene dichloride TiCl4 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Titanocene dichloride is the organotitanium compound with the formula (η5-C5H5)2TiCl2, commonly abbreviated as Cp2TiCl2. This metallocene is a common reagent in organometallic and organic synthesis. It exists as a bright red solid that slowly hydrolyzes in air.[2] It shows antitumour activity and was the first non-platinum complex to undergo clinical trials as a chemotherapy drug.[3]
Preparation and structure
The standard preparations of Cp2TiCl2 start with titanium tetrachloride. The original synthesis by Wilkinson and Birmingham, using sodium cyclopentadienide,[4] is still commonly used:[5]
- 2 NaC5H5 + TiCl4 → (C5H5)2TiCl2 + 2 NaCl
It can also be prepared by using freshly distilled cyclopentadiene rather than its sodium derivative:[6]
- 2 C5H6 + TiCl4 → (C5H5)2TiCl2 + 2 HCl
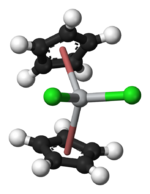
Focusing on the geometry of the Ti center, Cp2TiCl2 adopts a distorted tetrahedral geometry (counting Cp as a monodentate ligand). The Ti-Cl distance is 2.37 Å and the Cl-Ti-Cl angle is 95°.[7]
Reactions
Halide replacement reactions
Cp2TiCl2 serves as a source of Cp2Ti2+. A large range of nucleophiles will displace chloride. With NaSH and with polysulfide salts, one obtains the sulfido derivatives Cp2Ti(SH)2 and Cp2TiS5.[8]
The Petasis reagent, Cp2Ti(CH3)2, is prepared from the action of methylmagnesium chloride[9] or methyllithium[10] on Cp2TiCl2. This reagent is useful for the conversion of esters into vinyl ethers.
The Tebbe reagent Cp2TiCl(CH2)Al(CH3)2, arises by the action of 2 equivalents Al(CH3)3 on Cp2TiCl2.[11][12]
Reactions affecting Cp ligands
One Cp ligand can be removed from Cp2TiCl2 to give tetrahedral CpTiCl3. This conversion can be effected with TiCl4 or by reaction with SOCl2.[13]
The sandwich complex (Cycloheptatrienyl)(cyclopentadienyl)titanium is prepared by treatment of titanocene dichloride with lithium cycloheptatrienyl.[14]
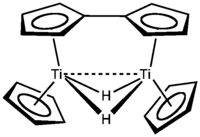
Titanocene itself, TiCp2, is so highly reactive that it rearranges into a TiIII hydride dimer and has been the subject of much investigation.[15][16] This dimer can be trapped by conducting the reduction of titanocene dichloride in the presence of ligands; in the presence of benzene, a fulvalene complex, μ(η5:η5-fulvalene)-di-(μ-hydrido)-bis(η5-cyclopentadienyltitanium), can be prepared and the resulting solvate structurally characterised by X-ray crystallography.[17] The same compound had been reported earlier by a lithium aluminium hydride reduction[18] and sodium amalgam reduction[19] of titanocene dichloride, and studied by 1H NMR[20] prior to its definitive characterisation.[15][16]
Reduction
Reduction with zinc gives the dimer of bis(cyclopentadienyl)titanium(III) chloride in a solvent-mediated chemical equilibrium:[21][22]
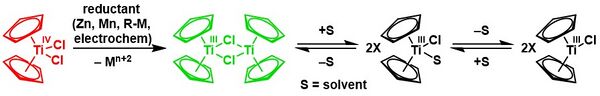
Cp2TiCl2 is a precursor to TiII derivatives. Reductions have been investigated using Grignard reagent and alkyl lithium compounds. More conveniently handled reductants include Mg, Al, or Zn. The following syntheses demonstrate some of the compounds that can be generated by reduction of titanocene dichloride in the presence of π acceptor ligands:[23]
- Cp2TiCl2 + 2 CO + Mg → Cp2Ti(CO)2 + MgCl2
- Cp2TiCl2 + 2 PR3 + Mg → Cp2Ti(PR3)2 + MgCl2
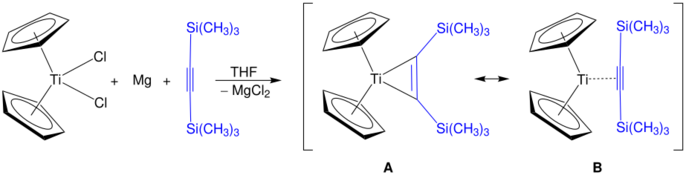
Alkyne derivatives of titanocene have the formula (C5H5)2Ti(C2R2) and the corresponding benzyne complexes are known.[24] One family of derivatives are the titanocyclopentadienes.[25] Rosenthal's reagent, Cp2Ti(η2-Me3SiC≡CSiMe3), can be prepared by this method. Two structures are shown, A and B, which are both resonance contributors to the actual structure of Rosenthal's reagent.[26]
Titanocene equivalents react with alkenyl alkynes followed by carbonylation and hydrolysis to form bicyclic cyclopentadienones, related to the Pauson–Khand reaction.[27] A similar reaction is the reductive cyclization of enones to form the corresponding alcohol in a stereoselective manner.[28]
Reduction of titanocene dichloride in the presence of conjugated dienes such as 1,3-butadiene gives η3-allyltitanium complexes.[29] Related reactions occur with diynes. Furthermore, titanocene can catalyze C–C bond metathesis to form asymmetric diynes.[25]
Derivatives of (C5Me5)2TiCl2
Many analogues of Cp2TiCl2 are known. Prominent examples are the ring-methylated derivatives (C5H4Me)2TiCl2 and (C5Me5)2TiCl2.
Catalysis
Titanium catalysts are an attractive from the perspective of green chemistry, i.e. the low toxicity and high abundance of titanium.[30]
Medicinal research
Titanocene dichloride was investigated as an anticancer drug. In fact, it was both the first non-platinum coordination complex and the first metallocene to undergo a clinical trial.[3][31]
References
- ↑ "Summary of Classification and Labelling". https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/41331.
- ↑ Budaver, S., ed (1989). The Merck Index (11th ed.). Merck & Co., Inc..
- ↑ 3.0 3.1 Roat-Malone, R. M. (2007). Bioinorganic Chemistry: A Short Course (2nd ed.). John Wiley & Sons. pp. 19–20. ISBN 978-0-471-76113-6. https://books.google.com/books?id=Ykx54y7LdK8C&q=molybdocene+dichloride&pg=PA20.
- ↑ Wilkinson, G.; Birmingham, J.G. (1954). "Bis-cyclopentadienyl Compounds of Ti, Zr, V, Nb and Ta". J. Am. Chem. Soc. 76 (17): 4281–4284. doi:10.1021/ja01646a008.
- ↑ Sara E. Johnson; Taylor A. Bell; Joseph K. West (2022). "Cp2TiCl2: Synthesis, Characterization, Modeling and Catalysis". Journal of Chemical Education 99 (5): 2121–2128. doi:10.1021/acs.jchemed.1c01272. Bibcode: 2022JChEd..99.2121J.
- ↑ Birmingham, J. M. (1965). "Synthesis of Cyclopentadienyl Metal Compounds". Adv. Organometal. Chem.. Advances in Organometallic Chemistry 2: 365–413. doi:10.1016/S0065-3055(08)60082-9. ISBN 9780120311026.
- ↑ Clearfield, Abraham et al. (1975). "Structural Studies of (π-C5H5)2MX2 Complexes and their Derivatives. The Structure of Bis(π-cyclopentadienyl)titanium Dichloride". Can. J. Chem. 53 (11): 1621–1629. doi:10.1139/v75-228.
- ↑ Shaver, Alan; McCall, James M.; Marmolejo, Gabriela (1990). "Cyclometallapolysulfanes (And Selanes) of Bis(η5-Cyclopentadienyl) Titanium(IV), Zirconium(IV), Molybdenum(IV), and Tungsten(IV)". Cyclometallapolysulfanes (and Selanes) of Bis(η5-Cyclopentadienyl) Titanium(IV), Zirconium(IV), Molybdenum(IV), and Tungsten(IV). Inorganic Syntheses. 27. pp. 59–65. doi:10.1002/9780470132586.ch11. ISBN 9780470132586.
- ↑ Payack, J. F.; Hughes, D. L.; Cai, D.; Cottrell, I. F.; Verhoeven, T. R. (2002). "Dimethyltitanocene". Organic Syntheses 79: 19. http://www.orgsyn.org/demo.aspx?prep=v79p0019.
- ↑ Claus, K.; Bestian, H. (1962). "Über die Einwirkung von Wasserstoff auf einige metallorganische Verbindungen und Komplexe". Justus Liebigs Ann. Chem. 654: 8–19. doi:10.1002/jlac.19626540103.
- ↑ Herrmann, W.A. (1982). "The Methylene Bridge". Adv. Organomet. Chem.. Advances in Organometallic Chemistry 20: 159–263. doi:10.1016/s0065-3055(08)60522-5. ISBN 9780120311200.
- ↑ Straus, D. A. (2000). "μ-Chlorobis(cyclopentadienyl)(dimethylaluminium)-μ-methylenetitanium". Encyclopedia of Reagents for Organic Synthesis. London: John Wiley.
- ↑ Chandra, K.; Sharma, R. K.; Kumar, N.; Garg, B. S. (1980). "Preparation of η5-Cyclopentadienyltitanium Trichloride and η5-Methylcyclopentadienyltitanium Trichloride". Chem. Ind. - London 44: 288–289.
- ↑ Camargo, Luana C.; Briganti, Matteo; Santana, Francielli S.; Stinghen, Danilo; Ribeiro, Ronny R.; Nunes, Giovana G.; Soares, Jaísa F.; Salvadori, Enrico et al. (2021). "Exploring the Organometallic Route to Molecular Spin Qubits: The [Cp Ti(cot)] Case". Angewandte Chemie International Edition 60 (5): 2588–2593. doi:10.1002/anie.202009634. PMID 33051985. https://zenodo.org/record/4926051.
- ↑ 15.0 15.1 Wailes, P. C.; Coutts, R. S. P.; Weigold, H. (1974). "Titanocene". Organometallic Chemistry of Titanium, Zirconium, and Hafnium. Academic Press. pp. 229–237. ISBN 9780323156479. https://books.google.com/books?id=wIlsA73iqpEC&pg=PA229.
- ↑ 16.0 16.1 16.2 Mehrotra, R. C.; Singh, A. (2000). "4.3.6 η5-Cyclopentadienyl d-Block Metal Complexes". Organometallic Chemistry: A Unified Approach (2nd ed.). New Delhi: New Age International Publishers. pp. 243–268. ISBN 9788122412581. https://books.google.com/books?id=NSQy3mFKRM8C&pg=PA258.
- ↑ 17.0 17.1 Troyanov, Sergei I.; Antropiusová, Helena; Mach, Karel (1992). "Direct proof of the molecular structure of dimeric titanocene; The X-ray structure of μ(η5:η5-fulvalene)-di-(μ-hydrido)-bis(η5-cyclopentadienyltitanium)·1.5 benzene". J. Organomet. Chem. 427 (1): 49–55. doi:10.1016/0022-328X(92)83204-U.
- ↑ Antropiusová, Helena; Dosedlová, Alena; Hanuš, Vladimir; Karel, Mach (1981). "Preparation of μ-(η5:η5-Fulvalene)-di-μ-hydrido-bis(η5-cyclopentadienyltitanium) by the reduction of Cp2TiCl2 with LiAlH4 in aromatic solvents". Transition Met. Chem. 6 (2): 90–93. doi:10.1007/BF00626113.
- ↑ Cuenca, Tomas; Herrmann, Wolfgang A.; Ashworth, Terence V. (1986). "Chemistry of oxophilic transition metals. 2. Novel derivatives of titanocene and zirconocene". Organometallics 5 (12): 2514–2517. doi:10.1021/om00143a019.
- ↑ Lemenovskii, D. A.; Urazowski, I. F.; Grishin, Yu K.; Roznyatovsky, V. A. (1985). "1H NMR Spectra and electronic structure of binuclear niobocene and titanocene containing fulvalene ligands". J. Organomet. Chem. 290 (3): 301–305. doi:10.1016/0022-328X(85)87293-4.
- ↑ Manzer, L. E.; Mintz, E. A.; Marks, T. J. (1982). "18. Cyclopentadienyl Complexes of Titanium(III) and Vanadium(III)". Inorganic Syntheses. 21. 84–86. doi:10.1002/9780470132524.ch18. ISBN 9780470132524.
- ↑ Nugent, William A.; RajanBabu, T. V. (1988). "Transition-metal-centered radicals in organic synthesis. Titanium(III)-induced cyclization of epoxy olefins". J. Am. Chem. Soc. 110 (25): 8561–8562. doi:10.1021/ja00233a051.
- ↑ Kuester, Erik (2002). "Bis(5-2,4-cyclopentadienyl)bis(trimethylphosphine)titanium". Encyclopedia of Reagents for Organic Synthesis. John Wiley. doi:10.1002/047084289X.rn00022. ISBN 0471936235.
- ↑ Buchwald, S. L.; Nielsen, R. B. (1988). "Group 4 Metal Complexes of Benzynes, Cycloalkynes, Acyclic Alkynes, and Alkenes". Chem. Rev. 88 (7): 1047–1058. doi:10.1021/cr00089a004.
- ↑ 25.0 25.1 Rosenthal, Uwe; Pellny, Paul-Michael; Kirchbauer, Frank G.; Burlakov, Vladimir V. (2000). "What Do Titano- and Zirconocenes Do with Diynes and Polyynes?". Chem. Rev. 33 (2): 119–129. doi:10.1021/ar9900109. PMID 10673320.
- ↑ Rosenthal, Uwe; Burlakov, Vladimir V.; Arndt, Perdita; Baumann, Wolfgang; Spannenberg, Anke (2003). "The Titanocene Complex of Bis(trimethylsilyl)acetylene: Synthesis, Structure, and Chemistry". Organometallics 22 (5): 884–900. doi:10.1021/om0208570.
- ↑ Hicks, F. A. (1999). "Scope of the Intramolecular Titanocene-Catalyzed Pauson-Khand Type Reaction". J. Am. Chem. Soc. 121 (25): 5881–5898. doi:10.1021/ja990682u.
- ↑ Kablaoui, N. M.; Buchwald, S. L. (1998). "Development of a Method for the Reductive Cyclization of Enones by a Titanium Catalyst". J. Am. Chem. Soc. 118 (13): 3182–3191. doi:10.1021/ja954192n.
- ↑ Sato, F.; Urabe, Hirokazu; Okamoto, Sentaro (2000). "Synthesis of Organotitanium Complexes from Alkenes and Alkynes and Their Synthetic Applications". Chem. Rev. 100 (8): 2835–2886. doi:10.1021/cr990277l. PMID 11749307.
- ↑ Anastas, Paul T.; Kirchhoff, Mary M. (2002-09-01). "Origins, Current Status, and Future Challenges of Green Chemistry" (in en). Accounts of Chemical Research 35 (9): 686–694. doi:10.1021/ar010065m. ISSN 0001-4842. PMID 12234198. https://pubs.acs.org/doi/10.1021/ar010065m.
- ↑ Cini, M.; Bradshaw, T. D.; Woodward, S. (2017). "Using titanium complexes to defeat cancer: the view from the shoulders of Titans". Chem. Soc. Rev. 46 (4): 1040–1051. doi:10.1039/C6CS00860G. PMID 28124046. http://eprints.nottingham.ac.uk/40186/1/ChemSocRev-ForNottsEPrints.pdf. Retrieved 2019-07-13.
Further reading
- Payack, J. F.; Hughes, D. L.; Cai, D.; Cottrell, I. F.; Verhoeven, T. R.. "Dimethyltitanocene Titanium, bis(η5-2,4-cyclopentadien-1-yl)dimethyl-". Organic Syntheses 79: 19. http://www.orgsyn.org/demo.aspx?prep=V79P0019.; Collective Volume, 10.
- Gambarotta, S.; Floriani, C.; Chiesi-Villa, A.; Guastini, C. (1983). "Cyclopentadienyldichlorotitanium(III): a free-radical-like reagent for reducing azo (N:N) multiple bonds in azo and diazo compounds". J. Am. Chem. Soc. 105 (25): 7295–7301. doi:10.1021/ja00363a015.
- Chirik, P. J. (2010). "Group 4 Transition Metal Sandwich Complexes: Still Fresh after Almost 60 Years". Organometallics 29 (7): 1500–1517. doi:10.1021/om100016p.
 |

