Chemistry:Ehrlich's reagent
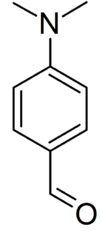
Ehrlich's reagent or Ehrlich reagent is a reagent containing p-dimethylaminobenzaldehyde (DMAB) and thus can act as an indicator to presumptively identify indoles and urobilinogen. Several Ehrlich tests use the reagent in a medical test; some are drug tests and others contribute to diagnosis of various diseases or adverse drug reactions. It is named after Nobel Prize winner Paul Ehrlich who used it to distinguish typhoid from simple diarrhoea.
The Ehrlich reagent works by binding to the C2 position of two indole moieties to form a resonance stabilised carbenium ion compound.[1]
Medical testing

Ehrlich reagent can be used to detect urobilinogen, which can indicate jaundice or other liver-related issues.
A very common Ehrlich test is a simple spot test to identify possible psychoactive compounds such as tryptamines (e.g. DMT) and lysergamides (e.g. LSD). It gives a negative test-result for 25I-NBOMe and many other non-indole-related psychoactives. The reagent will also give a positive result for opium, because of the presence of tryptophan in natural opium.[3]
Pyridoxine, present in vitamin supplements, can give positive results to the Ehrlich test, showing a pink colour change.[4]
Preparation
The reagent is prepared by dissolving 0.5[5]–2.0 g of p–dimethylaminobenzaldehyde (DMAB) in 50 mL of 95% ethanol and 50 mL of concentrated hydrochloric acid[6][7] and is best used when fresh. Other alcohols, such as 1-propanol, can also be used as well.[8]
The Ehrlich reagent is similar to a number of other indole tests:
- The van Urk reagent, which uses 0.125 g of p-DMAB, 0.2 mL of ferric chloride solution (25 g/mL) in 100 mL of 65% sulfuric acid.[9][10][11] This is sometimes referred to as the Hofmann reagent or p-DMAB-TS (Test Solution) and gives slightly different colours with different indoles.
- The Renz and Loew reagent, which uses p-dimethylaminocinnamaldehyde and may also be used for the detection of flavonoids.
- The "improved hallucinogen reagent", which uses a 1:1 solution of 5% DMAB in concentrated phosphoric acid (specific gravity 1.45) to methanol.[3][12]
See also
- Drug checking
- Other alkaloid spot tests:
- Froehde reagent
- Indole test
- Kovac's reagent, similar but uses isoamyl alcohol
- Liebermann reagent
- Marquis reagent
References
- ↑ "Chemistry and Reaction Mechanisms of Rapid Tests for Drugs of Abuse and Precursors Chemicals". UNODC. February 1989. pp. 15. https://www.unodc.org/pdf/scientific/SCITEC6.pdf.
- ↑ "Lysergide (LSD) drug profile". https://www.emcdda.europa.eu/publications/drug-profiles/lsd_en.
- ↑ 3.0 3.1 de Faubert Maunder, MJ (1975). "Field and laboratory test for raw and prepared opium.". Bulletin on Narcotics 27 (1): 71–6. PMID 1039285. http://www.unodc.org/unodc/en/data-and-analysis/bulletin/bulletin_1975-01-01_1_page008.html.
- ↑ "Pyridoxine Monograph for Professionals" (in en). https://www.drugs.com/monograph/pyridoxine.html.
- ↑ Spratley, Trinette (2004). "Analytical Profiles for Five "Designer" Tryptamines" (PDF). Microgram Journal 3 (1–2): 55. http://www.erowid.org/library/periodicals/microgram/microgram_journal_2005-1.pdf#page=54. Retrieved 2013-10-09.
- ↑ O’Neal, Carol L; Crouch, Dennis J; Fatah, Alim A (April 2000). "Validation of twelve chemical spot tests for the detection of drugs of abuse". Forensic Science International 109 (3): 189–201. doi:10.1016/S0379-0738(99)00235-2. PMID 10725655.
- ↑ "Color Test Reagents/Kits for Preliminary Identification of Drugs of Abuse". Law Enforcement and Corrections Standards and Testing Program. July 2000. http://www.ncjrs.gov/pdffiles1/nij/183258.pdf.
- ↑ "Ehrlich's Reagent Safety Data Sheet". Labchem. 2 July 2014. http://www.labchem.com/tools/msds/msds/LC14080.pdf.
- ↑ Ehmann, A. (1977). "The van URK-Salkowski reagent — a sensitive and specific chromogenic reagent for silica gel thin-layer chromatographic detection and identification of indole derivatives". Journal of Chromatography A 132 (2): 267–276. doi:10.1016/S0021-9673(00)89300-0. PMID 188858. https://erowid.org/psychoactives/testing/testing_field_article2.pdf.
- ↑ Sunshine, Irving (1969) (in en). Handbook of analytical toxicology. Chemical Rubber Co.. pp. 408. https://books.google.com/books?id=AEprAAAAMAAJ&q=%2522p-DMAB-TS%2522. "p-DMAB-TS: To a cool soln of 65 ml H2S04 in 35 ml H2O, add 125 mg para-dimethylaminobenzaldehyde, dissolve, add 1-2 drops of FeCI3-TS."
- ↑ "Basic Tests for Pharmaceutical Substances: 5. Reagents". https://apps.who.int/medicinedocs/en/d/Jh1803e/6.html.
- ↑ Maunder, M. J. de Faubert (August 1974). "A field test for hallucinogens: further improvements". Journal of Pharmacy and Pharmacology 26 (8): 637–638. doi:10.1111/j.2042-7158.1974.tb10677.x. PMID 4155730.
 |