Chemistry:Isoamyl alcohol
| |
| Names | |
|---|---|
| Preferred IUPAC name
3-Methylbutan-1-ol | |
| Other names
3-Methyl-1-butanol
Isopentyl alcohol Isopentanol Isobutylcarbinol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C5H12O | |
| Molar mass | 88.148 g/mol |
| Appearance | Clear, colorless liquid |
| Odor | Disagreeable odor in high concentrations |
| Density | 0.8104 g/cm3 at 20 °C |
| Melting point | −117[2][3] °C (−179 °F; 156 K) |
| Boiling point | 131.1 °C (268.0 °F; 404.2 K) |
| Slightly soluble, 28 g/L | |
| Solubility | Very soluble in acetone, diethyl ether, ethanol |
| Vapor pressure | 28 mmHg (20 °C)[3] |
| −68.96·10−6 cm3/mol | |
| Viscosity | 3.692 mPa·s |
| Thermochemistry | |
Heat capacity (C)
|
2.382 J/g·K |
Std enthalpy of
formation (ΔfH⦵298) |
−356.4 kJ/mol (liquid) −300.7 kJ/mol (gas) |
| Hazards | |
| Main hazards | Flammable, moderately toxic |
| GHS pictograms |   
|
| GHS Signal word | DANGER |
| H226, H302, H305, H315, H318, H332, H335 | |
| PP210Script error: No such module "Preview warning".Category:GHS errors, PP233Script error: No such module "Preview warning".Category:GHS errors, PP240Script error: No such module "Preview warning".Category:GHS errors, PP241Script error: No such module "Preview warning".Category:GHS errors, PP242Script error: No such module "Preview warning".Category:GHS errors, PP243Script error: No such module "Preview warning".Category:GHS errors, PP261Script error: No such module "Preview warning".Category:GHS errors, PP264Script error: No such module "Preview warning".Category:GHS errors, PP270Script error: No such module "Preview warning".Category:GHS errors, PP271Script error: No such module "Preview warning".Category:GHS errors, PP280Script error: No such module "Preview warning".Category:GHS errors, PP301+P312Script error: No such module "Preview warning".Category:GHS errors, PP302+P352Script error: No such module "Preview warning".Category:GHS errors, PP303+P361+P353Script error: No such module "Preview warning".Category:GHS errors, PP304+P312Script error: No such module "Preview warning".Category:GHS errors, PP304+P340Script error: No such module "Preview warning".Category:GHS errors, PP305+P351+P338Script error: No such module "Preview warning".Category:GHS errors, PP310Script error: No such module "Preview warning".Category:GHS errors, PP312Script error: No such module "Preview warning".Category:GHS errors, PP321Script error: No such module "Preview warning".Category:GHS errors, PP330Script error: No such module "Preview warning".Category:GHS errors, PP332+P313Script error: No such module "Preview warning".Category:GHS errors, PP337+P313Script error: No such module "Preview warning".Category:GHS errors, PP362Script error: No such module "Preview warning".Category:GHS errors, PP370+P378Script error: No such module "Preview warning".Category:GHS errors | |
| NFPA 704 (fire diamond) | |
| Flash point | 43 °C (109 °F; 316 K) |
| 350 °C (662 °F; 623 K) | |
| Explosive limits | 1.2–9% |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
1453 mg/kg (rabbit, oral) 1300 mg/kg (rat, oral)[4] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 100 ppm (360 mg/m3)[3] |
REL (Recommended)
|
TWA 100 ppm (360 mg/m3), ST 125 ppm (450 mg/m3)[3] |
IDLH (Immediate danger)
|
500 ppm[3] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
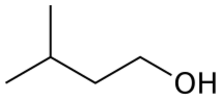
Isoamyl alcohol is a colorless liquid with the formula C5H12O, specifically (H3C–)2CH–CH2–CH2–OH. It is one of several isomers of amyl alcohol (pentanol). It is also known as isopentyl alcohol, isopentanol, or (in the IUPAC recommended nomenclature) 3-methyl-butan-1-ol. An obsolete name for it was isobutyl carbinol.[5]
Isoamyl alcohol is an ingredient in the production of banana oil, an ester found in nature and also produced as a flavouring in industry. It is a common fusel alcohol, produced as a major by-product of ethanol fermentation.
Occurrence
Isoamyl alcohol is one of the components of the aroma of Tuber melanosporum, the black truffle.
The compound has also been identified as a chemical in the pheromone used by hornets to attract other members of the hive to attack.[6]
Isoamyl acetate is a component of the natural aroma of bananas, especially the Gros Michel variety.
Extraction from fusel oil
Isoamyl alcohol can be separated from fusel oil by either of two methods: shaking with strong brine solution and separating the oily layer from the brine layer; distilling it and collecting the fraction that boils between 125 and 140 °C. Further purification is possible with this procedure: shaking the product with hot limewater, separating the oily layer, drying the product with calcium chloride, and distilling it, collecting the fraction boiling between 128 and 132 °C.[5]
Synthesis
Isoamyl alcohol can be synthesized by condensation of isobutene and formaldehyde which produces isoprenol and hydrogenation. It is a colourless liquid of density 0.8247 g/cm3 (0 °C), boiling at 131.6 °C, slightly soluble in water, and easily dissolved in organic solvents. It has a characteristic strong smell and a sharp burning taste. On passing the vapour through a red-hot tube, it decomposes into acetylene, ethylene, propylene, and other compounds. It is oxidized by chromic acid to isovaleraldehyde, and it forms addition compounds crystals with calcium chloride and tin(IV) chloride.[5]
Uses
Besides its use in the synthesis of banana oil, isoamyl alcohol is also an ingredient of Kovac's reagent, used for the bacterial diagnostic indole test.
It is also used as an antifoaming agent in the chloroform isoamyl alcohol reagent.[7]
Isoamyl alcohol is used in a phenol–chloroform extraction mixed with the chloroform to further inhibit RNase activity and prevent solubility of RNAs with long tracts of poly-adenine.[8]
Drugs
IAA is also used as the reactant in the synthesis of the following list of drugs:
- Amixetrine
- Amoproxan
- Camylofin
- Fenetradil
References
- ↑ Lide, David R., ed (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, Florida: CRC Press. pp. 3-374, 5-42, 6-188, 8-102, 15-22. ISBN 0-8493-0487-3.
- ↑ Straka, M.; van Genderen, A.; Růžička, K.; Růžička, V. Heat Capacities in the Solid and in the Liquid Phase of Isomeric Pentanols. J. Chem. Eng. Data 2007, 52, 794-802.
- ↑ 3.0 3.1 3.2 3.3 3.4 NIOSH Pocket Guide to Chemical Hazards. "#0348". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0348.html.
- ↑ "Isoamyl alcohol". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/idlh/123513.html.
- ↑ 5.0 5.1 5.2
 One or more of the preceding sentences incorporates text from a publication now in the public domain: Chisholm, Hugh, ed (1911). "Amyl Alcohols". Encyclopædia Britannica. 1 (11th ed.). Cambridge University Press. p. 900.
One or more of the preceding sentences incorporates text from a publication now in the public domain: Chisholm, Hugh, ed (1911). "Amyl Alcohols". Encyclopædia Britannica. 1 (11th ed.). Cambridge University Press. p. 900.
- ↑ Wilson, Calum & Davies, Noel & Corkrey, Ross & J. Wilson, Annabel & M. Mathews, Alison & C. Westmore, Guy. (2017). Receiver Operating Characteristic curve analysis determines association of individual potato foliage volatiles with onion thrips preference, cultivar and plant age. PLOS ONE. 12. e0181831. 10.1371/journal.pone.0181831.
- ↑ Zumbo, P.. "Phenol-chloroform Extraction". WEILL CORNELL MEDICAL COLLEGE P. ZUMBO LABORATORY OF CHRISTOPHER E. MASON, PH.D. http://physiology.med.cornell.edu/faculty/mason/lab/zumbo/files/PHENOL-CHLOROFORM.pdf.
- ↑ Green, Michael; Sambrook, Joseph. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press. ISBN 1936113422. http://www.molecularcloning.com/.
 |


