Chemistry:Glucose 1-phosphate
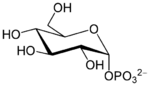 Anionic form of α-D-glucose 1-phosphate
| |
 Neutral form of α-D-glucose 1-phosphate
| |
| Names | |
|---|---|
| IUPAC name
D-Glucopyranosyl dihydrogen phosphate
| |
| Systematic IUPAC name
(2Ξ,3R,4S,5S,6R)-3,4,5-Trihydroxy-6-(hydroxymethyl)oxan-2-yl dihydrogen phosphate | |
| Other names
Cori ester
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
| MeSH | glucose-1-phosphate |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H13O9P | |
| Molar mass | 260.135 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Glucose 1-phosphate (also called Cori ester) is a glucose molecule with a phosphate group on the 1'-carbon. It can exist in either the α- or β-anomeric form.
Reactions of α-glucose 1-phosphate
Catabolic
In glycogenolysis, it is the direct product of the reaction in which glycogen phosphorylase cleaves off a molecule of glucose from a greater glycogen structure. A deficiency of muscle glycogen phosphorylase is known as glycogen storage disease type V (McArdle Disease).
To be utilized in cellular catabolism it must first be converted to glucose 6-phosphate by the enzyme phosphoglucomutase in a free equilibrium.[1][2][3] One reason that cells form glucose 1-phosphate instead of glucose during glycogen breakdown is that the very polar phosphorylated glucose cannot leave the cell membrane and so is marked for intracellular catabolism. Phosphoglucomutase-1 deficiency is known as glycogen storage disease type 14 (GSD XIV).[4]
Anabolic
In glycogenesis, free glucose 1-phosphate can also react with UTP to form UDP-glucose,[5] by using the enzyme UDP-glucose pyrophosphorylase. It can then return to the greater glycogen structure via glycogen synthase.[5]
β-Glucose 1-phosphate
β-Glucose 1-phosphate is found in some microbes. It is produced by inverting α-glucan phosphorylases including maltose phosphorylase, kojibiose phosphorylase and trehalose phosphorylase and is then converted into glucose 6-phosphate by β-phosphoglucomutase.
See also
References
- ↑ Pelley, John W. (2012-01-01), Pelley, John W., ed., "8 - Gluconeogenesis and Glycogen Metabolism" (in en), Elsevier's Integrated Review Biochemistry (Second Edition) (Philadelphia: W.B. Saunders): pp. 67–73, doi:10.1016/b978-0-323-07446-9.00008-8, ISBN 978-0-323-07446-9, http://www.sciencedirect.com/science/article/pii/B9780323074469000088, retrieved 2020-12-16
- ↑ Isselbacher, Kurt J. (1965-01-01), Bergmeyer, Hans-Ulrich, ed., "Galactose-1-phosphate Uridyl Transferase" (in en), Methods of Enzymatic Analysis (Academic Press): pp. 863–866, doi:10.1016/b978-0-12-395630-9.50153-5, ISBN 978-0-12-395630-9, http://www.sciencedirect.com/science/article/pii/B9780123956309501535, retrieved 2020-12-16
- ↑ Bergmeyer, Hans-Ulrich; Klotzsch, Helmut (1965-01-01), Bergmeyer, Hans-Ulrich, ed., "d-Glucose-1-phosphate" (in en), Methods of Enzymatic Analysis (Academic Press): pp. 131–133, doi:10.1016/b978-0-12-395630-9.50024-4, ISBN 978-0-12-395630-9, http://www.sciencedirect.com/science/article/pii/B9780123956309500244, retrieved 2020-12-16
- ↑ Orphanet: Glycogen storage disease due to phosphoglucomutase deficiency
- ↑ 5.0 5.1 Blanco, Antonio; Blanco, Gustavo (2017-01-01), Blanco, Antonio; Blanco, Gustavo, eds., "Chapter 19 - Integration and Regulation of Metabolism" (in en), Medical Biochemistry (Academic Press): pp. 425–445, doi:10.1016/b978-0-12-803550-4.00019-7, ISBN 978-0-12-803550-4, http://www.sciencedirect.com/science/article/pii/B9780128035504000197, retrieved 2020-12-16
 |

