Chemistry:Triethanolamine
| |
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
2,2′,2′′-Nitrilotri(ethan-1-ol)[1] | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| 3DMet | |
| 1699263 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
| MeSH | Biafine |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| N(CH 2CH 2OH) 3 | |
| Molar mass | 149.190 g·mol−1 |
| Appearance | Colourless, viscous liquid |
| Odor | Ammoniacal |
| Density | 1.124 g/mL |
| Melting point | 21.60 °C; 70.88 °F; 294.75 K |
| Boiling point | 335.40 °C; 635.72 °F; 608.55 K |
| miscible | |
| log P | −0.988 |
| Vapor pressure | 1 Pa (at 20 °C) |
| Acidity (pKa) | 7.74[2] |
| UV-vis (λmax) | 280 nm |
Refractive index (nD)
|
1.485 |
| Thermochemistry | |
Heat capacity (C)
|
389 J K−1 mol−1 |
Std enthalpy of
formation (ΔfH⦵298) |
−665.7 – −662.7 kJ mol−1 |
Std enthalpy of
combustion (ΔcH⦵298) |
−3.8421 – −3.8391 MJ mol−1 |
| Pharmacology | |
| 1=ATC code }} | D03AX12 (WHO) |
| Hazards | |
| Safety data sheet | hazard.com |
| GHS pictograms | 
|
| GHS Signal word | WARNING |
| H319 | |
| P305+351+338 | |
| NFPA 704 (fire diamond) | |
| Flash point | 179 °C (354 °F; 452 K) |
| 325 °C (617 °F; 598 K) | |
| Explosive limits | 1.3–8.5% |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
|
| Related compounds | |
Related alkanols
|
|
Related compounds
|
Diethylhydroxylamine |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
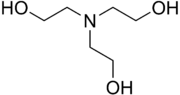
Triethanolamine, or TEOA, is an organic compound with the chemical formula N(CH
2CH
2OH)
3. It is a colourless, viscous liquid. It is both a tertiary amine and a triol. A triol is a molecule with three alcohol groups. Approximately 150,000 tonnes were produced in 1999.[3] It is a colourless compound although samples may appear yellow because of impurities.
Production
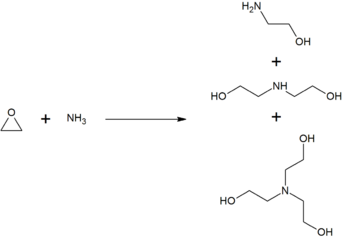
Triethanolamine is produced from the reaction of ethylene oxide with aqueous ammonia, also produced are ethanolamine and diethanolamine. The ratio of the products can be controlled by changing the stoichiometry of the reactants.[4]
Applications
Triethanolamine is used primarily in making surfactants, such as for emulsifier. It is a common ingredient in formulations used for both industrial and consumer products. The triethanolamine neutralizes fatty acids, adjusts and buffers the pH, and solubilizes oils and other ingredients that are not completely soluble in water. Triethanolammonium salts in some cases are more soluble than salts of alkali metals that might be used otherwise, and results in less alkaline products than would from using alkali metal hydroxides to form the salt. Some common products in which triethanolamine is found are sunscreen lotions, liquid laundry detergents, dishwashing liquids, general cleaners, hand sanitizers, polishes, metalworking fluids, paints, shaving cream and printing inks.[5]
Cement production
Triethanolamine is also used as organic additive (0.1 wt%) in the grinding of cement clinker. It facilitates the grinding process by preventing agglomeration and coating of the powder at the surface of balls and mill wall.[6]
Cosmetics and medicine
Various ear diseases and infections are treated with eardrops containing triethanolamine polypeptide oleate-condensate, such as Cerumenex in the United States. In pharmaceutics, triethanolamine is the active ingredient of some eardrops used to treat impacted earwax. It also serves as a pH balancer in many different cosmetic products, ranging from cleansing creams and milks, skin lotions, eye gels, moisturizers, shampoos, shaving foams, TEA is a fairly strong base: a 1% solution has a pH of approximately 10, whereas the pH of skin is less than pH 7, approximately 5.5−6.0. Cleansing milk–cream emulsions based on TEA are particularly good at removing makeup.
Derivatives
- Amustaline
- Trolnitrate
- Trimustine
In the laboratory and in amateur photography
Another common use of TEA is as a complexing agent for aluminium ions in aqueous solutions. This reaction is often used to mask such ions before complexometric titrations with another chelating agent such as EDTA. TEA has also been used in photographic (silver halide) processing. It has been promoted as a useful alkali by amateur photographers.
In holography
TEA is used to provide a sensitivity boost to silver-halide-based holograms, and also as a swelling agent to color shift holograms. It is possible to get the sensitivity boost without color shift by rinsing out the TEA before squeegee and drying.[7]
In electroless plating
TEA is now commonly and very effectively used as a complexing agent in electroless plating.
In ultrasonic testing
2-3% in water TEA is used as an corrosion inhibitor (anti-rust) agent in immersion ultrasonic testing.
In aluminium soldering
Triethanolamine, diethanolamine and aminoethylethanolamine are major components of common liquid organic fluxes for the soldering of aluminium alloys using tin-zinc and other tin or lead-based soft solders.[8][9][10]
Safety and regulation
Allergic reactions
A 1996 study found that triethanolamine (TEOA) occasionally causes contact allergy.[11] A 2001 study found TEOA in a sunscreen caused an allergic contact dermatitis.[12] A 2007 study found TEOA in ear drops caused a contact allergy.[13] Systemic and respiratory tract (RT) toxicity was analyzed for 28 days in a nose specific inhalation 2008 study in Wistar rats; TEOA seems to be less potent in regard to systemic toxicity and RT irritancy than diethanolamine (DEA). Exposure to TEOA resulted in focal inflammation, starting in single male animals from 20 mg/m3 concentrations.[14]
A 2009 study stated that patch test reactions reveal a slight irritant potential instead of a true allergic response in several cases, and also indicated the risk of skin sensitization to TEOA seems to be very low.[15]
Tumors
Reports indicated that TEOA causes an increased incidence of tumor growth in the liver in female B6C3F1 mice, but not in male mice or in Fischer 344 rats.[16] A 2004 study concluded "TEOA may cause liver tumors in mice via a choline-depletion mode of action and that this effect is likely caused by the inhibition of choline uptake by cells."[16]
Environmental toxicity
A 2009 study found that TEOA has potential acute, sub-chronic and chronic toxicity properties in respect to aquatic species.[17]
Regulation
TEOA is listed under Schedule 3, part B of the Chemical Weapons Convention as it can be used in the manufacture of HN3 nitrogen mustard.
See also
References
- ↑ "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. pp. P001–P004. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ↑ Simond, M. R. (2012). "Dissociation Constants of Protonated Amines in Water at Temperatures from 293.15 K to 343.15 K". Journal of Solution Chemistry 41: 130. doi:10.1007/s10953-011-9790-3.
- ↑ Frauenkron, Matthias; Melder, Johann-Peter; Ruider, Günther; Rossbacher, Roland; Höke, Hartmut. "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a10_001.
- ↑ Weissermel, Klaus; Arpe, Hans-Jürgen; Lindley, Charlet R.; Hawkins, Stephen (2003). "Chapter 7. Oxidation Products of Ethylene". Industrial Organic Chemistry. Wiley-VCH. pp. 159–161. ISBN 978-3-527-30578-0.
- ↑ Ashford, Robert D. (2011). Ashford's Dictionary of Industrial Chemicals (3rd ed.). Saltash, Cornwall: Wavelength Publications. p. 9252. ISBN 978-0-9522674-3-0.
- ↑ Sohoni, S.; Sridhar, R.; Mandal, G. (1991). "Effect of grinding aids on the fine grinding of limestone, quartz and portland cement clinker". Powder Technology 67 (3): 277–286. doi:10.1016/0032-5910(91)80109-V.
- ↑ "Holoforum.org". http://holoforum.org/oldforum/viewtopic.php?f=17&t=5058.
- ↑ "Kapp Liquid Flux SDS". https://www.kappalloy.com/pub/media/pdf/SDS_551_GHS_R12_Liquid_Golden_FLux.pdf.
- ↑ "Harris Stay-Clean Aluminum Flux SDS". http://www.lincolnelectric.com/assets/US/EN/MSDS_lib/ZLE_SDS_NA-EN-200000007463.pdf.
- ↑ "Superior #1260 Flux SDS". http://www.superiorflux.com/sds/1260.pdf.
- ↑ Hamilton, T. K.; Zug, K. A. (1996). "Triethanolamine allergy inadvertently discovered from a fluorescent marking pen". Am. J. Contact Dermat. 7 (3): 164–5. doi:10.1016/S1046-199X(96)90006-8. PMID 8957332.
- ↑ Chu, C. Y.; Sun, C. C. (2001). "Allergic contact dermatitis from triethanolamine in a sunscreen". Contact Dermatitis 44 (1): 41–2. doi:10.1034/j.1600-0536.2001.440107-8.x. PMID 11156016.
- ↑ Schmutz, J. L.; Barbaud, A.; Tréchot, P. (2007). "Contact allergy to triethanolamine in ear drops and shampoo". Ann. Dermatol. Venereol. 134 (1): 105. doi:10.1016/S0151-9638(07)89009-0. PMID 17384563.
- ↑ Gamer, A. O.; Rossbacher, R.; Kaufmann, W.; van Ravenzwaay, B. (2008). "The inhalation toxicity of di- and triethanolamine upon repeated exposure". Food Chem. Toxicol. 46 (6): 2173–2183. doi:10.1016/j.fct.2008.02.020. PMID 18420328.
- ↑ Lessmann, H.; Uter, W.; Schnuch, A.; Geier, J. (2009). "Skin sensitizing properties of the ethanolamines mono-, di-, and triethanolamine. Data analysis of a multicentre surveillance network (IVDK*) and review of the literature". Contact Dermatitis 60 (5): 243–255. doi:10.1111/j.1600-0536.2009.01506.x. PMID 19397616.
- ↑ 16.0 16.1 Stott, W. T.; Radtke, B. J.; Linscombe, V. A.; Mar, M. H.; Zeisel, S. H. (2004). "Evaluation of the potential of triethanolamine to alter hepatic choline levels in female B6C3F1 mice". Toxicol. Sci. 79 (2): 242–7. doi:10.1093/toxsci/kfh115. PMID 15056812.
- ↑ Libralato, G.; Volpi Ghirardini, A.; Avezzù, F. (2009). "Seawater ecotoxicity of monoethanolamine, diethanolamine and triethanolamine". J. Hazard. Mater. 176 (1–3): 535–9. doi:10.1016/j.jhazmat.2009.11.062. PMID 20022426.
 |