Biology:Toxin-antitoxin system
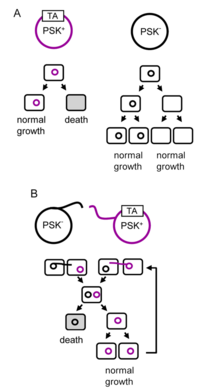
A toxin-antitoxin system consists of a "toxin" and a corresponding "antitoxin", usually encoded by closely linked genes. The toxin is usually a protein while the antitoxin can be a protein or an RNA. Toxin-antitoxin systems are widely distributed in prokaryotes, and organisms often have them in multiple copies.[2][3] When these systems are contained on plasmids – transferable genetic elements – they ensure that only the daughter cells that inherit the plasmid survive after cell division. If the plasmid is absent in a daughter cell, the unstable antitoxin is degraded and the stable toxic protein kills the new cell; this is known as 'post-segregational killing' (PSK).[4][5]
Toxin-antitoxin systems are typically classified according to how the antitoxin neutralises the toxin. In a type I toxin-antitoxin system, the translation of messenger RNA (mRNA) that encodes the toxin is inhibited by the binding of a small non-coding RNA antitoxin that binds the toxin mRNA. The toxic protein in a type II system is inhibited post-translationally by the binding of an antitoxin protein. Type III toxin-antitoxin systems consist of a small RNA that binds directly to the toxin protein and inhibits its activity.[6] There are also types IV-VI, which are less common.[7] Toxin-antitoxin genes are often inherited through horizontal gene transfer[8][9] and are associated with pathogenic bacteria, having been found on plasmids conferring antibiotic resistance and virulence.[1]
Chromosomal toxin-antitoxin systems also exist, some of which are thought to perform cell functions such as responding to stresses, causing cell cycle arrest and bringing about programmed cell death.[1][10] In evolutionary terms, toxin-antitoxin systems can be considered selfish DNA in that the purpose of the systems are to replicate, regardless of whether they benefit the host organism or not. Some have proposed adaptive theories to explain the evolution of toxin-antitoxin systems; for example, chromosomal toxin-antitoxin systems could have evolved to prevent the inheritance of large deletions of the host genome.[11] Toxin-antitoxin systems have several biotechnological applications, such as maintaining plasmids in cell lines, targets for antibiotics, and as positive selection vectors.[12]
Biological functions
Stabilization and fitness of mobile DNA
As stated above, toxin-antitoxin systems are well characterized as plasmid addiction modules. It was also proposed that toxin-antitoxin systems have evolved as plasmid exclusion modules. A cell that would carry two plasmids from the same incompatibility group will eventually generate two daughters cells carrying either plasmid. Should one of these plasmids encode for a TA system, its "displacement" by another TA-free plasmid system will prevent its inheritance and thus induce post-segregational killing.[13] This theory was corroborated through computer modelling.[14] Toxin-antitoxin systems can also be found on other mobile genetic elements such as conjugative transposons and temperate bacteriophages and could be implicated in the maintenance and competition of these elements.[15]
Genome stabilization
Toxin-antitoxin systems could prevent harmful large deletions in a bacterial genome, though arguably deletions of large coding regions are fatal to a daughter cell regardless.[11] In Vibrio cholerae, multiple type II toxin-antitoxin systems located in a super-integron were shown to prevent the loss of gene cassettes.[18]
Altruistic cell death
mazEF, a toxin-antitoxin locus found in E. coli and other bacteria, was proposed to induce programmed cell death in response to starvation, specifically a lack of amino acids.[19] This would release the cell's contents for absorption by neighbouring cells, potentially preventing the death of close relatives, and thereby increasing the inclusive fitness of the cell that perished. This would be an example of altruism and how bacterial colonies could resemble multicellular organisms.[14] However, the "mazEF-mediated PCD" has largely been refuted by several studies.[20][21][22]
Stress tolerance
Another theory states that chromosomal toxin-antitoxin systems are designed to be bacteriostatic rather than bactericidal.[23] RelE, for example, is a global inhibitor of translation, is induced during nutrient stress. By shutting down translation under stress, it could reduce the chance of starvation by lowering the cell's nutrient requirements.[24] However, it was shown that several toxin-antitoxin systems, including relBE, do not give any competitive advantage under any stress condition.[21]
Anti-addiction
It has been proposed that chromosomal homologues of plasmid toxin-antitoxin systems may serve as anti-addiction modules, which would allow progeny to lose a plasmid without suffering the effects of the toxin it encodes.[9] For example, a chromosomal copy of the ccdA antitoxin encoded in the chromosome of Erwinia chrysanthemi is able to neutralize the ccdB toxin encoded on the F plasmid and thus, prevent toxin activation when such a plasmid is lost.[25] Similarly, the ataR antitoxin encoded on the chromosome of E. coli O157:H7 is able neutralize the ataTP toxin encoded on plasmids found in other enterohemorragic E. coli.[26]
Phage protection
Type III toxin-antitoxin (AbiQ) systems have been shown to protect bacteria from bacteriophages altruistically.[27][28] During an infection, bacteriophages hijack transcription and translation, which could prevent antitoxin replenishment and release toxin, triggering what is called an "abortive infection".[27][28] Similar protective effects have been observed with type I,[29] type II,[30] and type IV (AbiE)[31] toxin-antitoxin systems.
Abortive initiation (Abi) can also happen without toxin-antitoxin systems, and many Abi proteins of other types exist. This mechanism serves to halt the replication of phages, protecting the overall population from harm.[32]
Antimicrobial persistence
When bacteria are challenged with antibiotics, a small and distinct subpopulation of cells is able to withstand the treatment by a phenomenon dubbed as "persistence" (not to be confused with resistance).[33] Due to their bacteriostatic properties, type II toxin-antitoxin systems have previously been thought to be responsible for persistence, by switching a fraction of the bacterial population to a dormant state.[34] However, this hypothesis has been widely invalidated.[35][36][37]
Selfish DNA
Toxin-antitoxin systems have been used as examples of selfish DNA as part of the gene centered view of evolution. It has been theorised that toxin-antitoxin loci serve only to maintain their own DNA, at the expense of the host organism.[1][38] Thus, chromosomal toxin-antitoxin systems would serve no purpose and could be treated as "junk DNA". For example, the ccdAB system encoded in the chromosome of E. coli O157:H7 has been shown to be under negative selection, albeit at a slow rate due to its addictive properties.[8]
System types
Type I
Type I toxin-antitoxin systems rely on the base-pairing of complementary antitoxin RNA with the toxin mRNA. Translation of the mRNA is then inhibited either by degradation via RNase III or by occluding the Shine-Dalgarno sequence or ribosome binding site of the toxin mRNA. Often the toxin and antitoxin are encoded on opposite strands of DNA. The 5' or 3' overlapping region between the two genes is the area involved in complementary base-pairing, usually with between 19–23 contiguous base pairs.[39]
Toxins of type I systems are small, hydrophobic proteins that confer toxicity by damaging cell membranes.[1] Few intracellular targets of type I toxins have been identified, possibly due to the difficult nature of analysing proteins that are poisonous to their bacterial hosts.[10] Also, the detection of small proteins has been challenging due to technical issues, a problem that remains to be solved with large-scale analysis.[40]
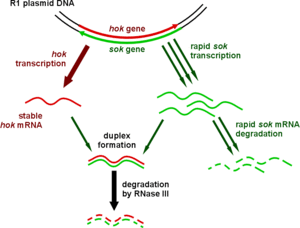
Type I systems sometimes include a third component. In the case of the well-characterised hok/sok system, in addition to the hok toxin and sok antitoxin, there is a third gene, called mok. This open reading frame almost entirely overlaps that of the toxin, and the translation of the toxin is dependent on the translation of this third component.[5] Thus the binding of antitoxin to toxin is sometimes a simplification, and the antitoxin in fact binds a third RNA, which then affects toxin translation.[39]
Example systems
| Toxin | Antitoxin | Notes | Ref. |
|---|---|---|---|
| hok | sok | The original and best-understood type I toxin-antitoxin system (pictured), which stabilises plasmids in a number of gram-negative bacteria | [39] |
| fst | RNAII | The first type I system to be identified in gram-positive bacteria | [41] |
| tisB | istR | A chromosomal system induced in the SOS response | [42] |
| dinQ | agrB | A chromosomal system induced in the SOS response | [43] |
| ldrD | rdlD | A chromosomal system in Enterobacteriaceae | [44] |
| flmA | flmB | A hok/sok homologue, which also stabilises the F plasmid | [45] |
| ibs | sib | Discovered in E. coli intergenic regions, the antitoxin was originally named QUAD RNA | [46] |
| txpA/brnT | ratA | Ensures the inheritance of the skin element during sporulation in Bacillus subtilis | [47] |
| symE | symR | A chromosomal system induced in the SOS response | [3] |
| XCV2162 | ptaRNA1 | A system identified in Xanthomonas campestris with erratic phylogenetic distribution. | [48] |
| timP | timR | A chromosomal system identified in Salmonella | [49] |
| aapA1 | isoA1 | A type 1 TA module in Helicobacter pylori | [50] |
| sprA1 | sprA1as | Located within S. aureus small Pathogenicity island (SaPI). SprA1 encodes for a small cytotoxic peptide, PepA1, which disrupts both S. aureus membranes and host erythrocytes. | [51][52] |
Type II
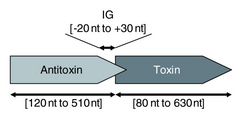
Type II toxin-antitoxin systems are generally better-understood than type I.[39] In this system a labile proteic antitoxin tightly binds and inhibits the activity of a stable toxin.[10] The largest family of type II toxin-antitoxin systems is vapBC,[53] which has been found through bioinformatics searches to represent between 37 and 42% of all predicted type II loci.[16][17] Type II systems are organised in operons with the antitoxin protein typically being located upstream of the toxin, which helps to prevent expression of the toxin without the antitoxin.[54] The proteins are typically around 100 amino acids in length,[39] and exhibit toxicity in a number of ways: CcdB, for example, affects DNA replication by poisoning DNA gyrase[55] whereas toxins from the MazF family are endoribonucleases that cleave cellular mRNAs,[56][57] tRNAs [58][59] or rRNAs [60] at specific sequence motifs. The most common toxic activity is the protein acting as an endonuclease, also known as an interferase.[61][62]
One of the key features of the TAs is the autoregulation. The antitoxin and toxin protein complex bind to the operator that is present upstream of the TA genes. This results in repression of the TA operon. The key to the regulation are (i) the differential translation of the TA proteins and (ii) differential proteolysis of the TA proteins. As explained by the "Translation-reponsive model",[63] the degree of expression is inversely proportional to the concentration of the repressive TA complex. The TA complex concentration is directly proportional to the global translation rate. The higher the rate of translation more TA complex and less transcription of TA mRNA. Lower the rate of translation, lesser the TA complex and higher the expression. Hence, the transcriptional expression of TA operon is inversely proportional to translation rate.
A third protein can sometimes be involved in type II toxin-antitoxin systems. in the case of the ω-ε-ζ (omega-epsilon-zeta) system, the omega protein is a DNA binding protein that negatively regulates the transcription of the whole system.[64] Similarly, the paaR2 protein regulates the expression of the paaR2-paaA2-parE2 toxin-antitoxin system.[65] Other toxin-antitoxin systems can be found with a chaperone as a third component.[66] This chaperone is essential for proper folding of the antitoxin, thus making the antitoxin addicted to its cognate chaperone.
Example systems
| Toxin | Antitoxin | Notes | Ref. |
|---|---|---|---|
| ccdB | ccdA | Found on the F plasmid of Escherichia coli | [55] |
| parE | parD | Found in multiple copies in Caulobacter crescentus | [67] |
| mazF | mazE | Found in E. coli and in chromosomes of other bacteria | [29] |
| yafO | yafN | A system induced by the SOS response to DNA damage in E. coli | [68] |
| hicA | hicB | Found in archaea and bacteria | [69] |
| kid | kis | Stabilises the R1 plasmid and is related to the CcdB/A system | [23] |
| ζ | ε | Found mostly in Gram-positive bacteria | [64] |
| ataT | ataR | Found in enterohemorragic E. coli and Klebsiella spp. | [70] |
Type III
| ToxN_toxin | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | ToxN, type III toxin-antitoxin system | ||||||||
| Pfam | PF13958 | ||||||||
| |||||||||
Type III toxin-antitoxin systems rely on direct interaction between a toxic protein and an RNA antitoxin. The toxic effects of the protein are neutralised by the RNA gene.[6] One example is the ToxIN system from the bacterial plant pathogen Erwinia carotovora. The toxic ToxN protein is approximately 170 amino acids long and has been shown to be toxic to E. coli. The toxic activity of ToxN is inhibited by ToxI RNA, an RNA with 5.5 direct repeats of a 36 nucleotide motif (AGGTGATTTGCTACCTTTAAGTGCAGCTAGAAATTC).[27][71] Crystallographic analysis of ToxIN has found that ToxN inhibition requires the formation of a trimeric ToxIN complex, whereby three ToxI monomers bind three ToxN monomers; the complex is held together by extensive protein-RNA interactions.[72]
Type IV
Type IV toxin-antitoxin systems are similar to type II systems, because they consist of two proteins. Unlike type II systems, the antitoxin in type IV toxin-antitoxin systems counteracts the activity of the toxin, and the two proteins do not necessarily interact directly. DarTG1 and DarTG2 are type IV toxin-antitoxin systems that modify DNA. Their toxins add ADP-ribose to guanosine bases (DarT1 toxin) or thymidine bases (DarT2 toxin), and their antitoxins remove the toxic modifications (NADAR antitoxin from guanosine and DarG antitoxin from thymidine).[73][74][75][76]
Type V
ghoST is a type V toxin-antitoxin system, in which the antitoxin (GhoS) cleaves the ghoT mRNA. This system is regulated by a type II system, mqsRA.[77]
Type VI
socAB is a type VI toxin-antitoxin system that was discovered in Caulobacter crescentus. The antitoxin, SocA, promotes degradation of the toxin, SocB, by the protease ClpXP.[78]
Type VII
Type VII has been proposed to include systems hha/tomB, tglT/takA and hepT/mntA, all of which neutralise toxin activity by post-translational chemical modification of amino acid residues.[79]
Type VIII
Type VIII includes the system creTA. In this system, the antitoxin creA serves as a guide RNA for a CRISPR-Cas system. Due to incomplete complementarity between the creA guide and the creAT promoter, the Cas complex does not cleave the DNA, but instead remains at the site, where it blocks access by RNA polymerase, preventing expression of the creT toxin (a natural instance of CRISPRi). When expressed, the creT RNA will sequester the rare arginine codon tRNAUCU, stalling translation and halting cell metabolism.[80]
Biotechnological applications
The biotechnological applications of toxin-antitoxin systems have begun to be realised by several biotechnology organisations.[12][23] A primary usage is in maintaining plasmids in a large bacterial cell culture. In an experiment examining the effectiveness of the hok/sok locus, it was found that segregational stability of an inserted plasmid expressing beta-galactosidase was increased by between 8 and 22 times compared to a control culture lacking a toxin-antitoxin system.[81][82] In large-scale microorganism processes such as fermentation, progeny cells lacking the plasmid insert often have a higher fitness than those who inherit the plasmid and can outcompete the desirable microorganisms. A toxin-antitoxin system maintains the plasmid thereby maintaining the efficiency of the industrial process.[12]
Additionally, toxin-antitoxin systems may be a future target for antibiotics. Inducing suicide modules against pathogens could help combat the growing problem of multi-drug resistance.[83]
Ensuring a plasmid accepts an insert is a common problem of DNA cloning. Toxin-antitoxin systems can be used to positively select for only those cells that have taken up a plasmid containing the inserted gene of interest, screening out those that lack the inserted gene. An example of this application comes from the ccdB-encoded toxin, which has been incorporated into plasmid vectors.[84] The gene of interest is then targeted to recombine into the ccdB locus, inactivating the transcription of the toxic protein. Thus, cells containing the plasmid but not the insert perish due to the toxic effects of CcdB protein, and only those that incorporate the insert survive.[12]
Another example application involves both the CcdB toxin and CcdA antitoxin. CcdB is found in recombinant bacterial genomes and an inactivated version of CcdA is inserted into a linearised plasmid vector. A short extra sequence is added to the gene of interest that activates the antitoxin when the insertion occurs. This method ensures orientation-specific gene insertion.[84]
Genetically modified organisms must be contained in a pre-defined area during research.[83] Toxin-antitoxin systems can cause cell suicide in certain conditions, such as a lack of a lab-specific growth medium they would not encounter outside of the controlled laboratory set-up.[23][85]
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 "Bacterial toxin-antitoxin systems: more than selfish entities?". PLOS Genetics 5 (3): e1000437. March 2009. doi:10.1371/journal.pgen.1000437. PMID 19325885.
- ↑ "Abundance of type I toxin-antitoxin systems in bacteria: searches for new candidates and discovery of novel families". Nucleic Acids Research 38 (11): 3743–59. June 2010. doi:10.1093/nar/gkq054. PMID 20156992.
- ↑ 3.0 3.1 "RNA antitoxins". Current Opinion in Microbiology 10 (2): 117–24. April 2007. doi:10.1016/j.mib.2007.03.003. PMID 17376733.
- ↑ "Toxin-antitoxin modules may regulate synthesis of macromolecules during nutritional stress". Journal of Bacteriology 182 (3): 561–72. February 2000. doi:10.1128/JB.182.3.561-572.2000. PMID 10633087.
- ↑ 5.0 5.1 "Competitive inhibition of natural antisense Sok-RNA interactions activates Hok-mediated cell killing in Escherichia coli". Nucleic Acids Research 34 (20): 5915–22. 2006. doi:10.1093/nar/gkl750. PMID 17065468.
- ↑ 6.0 6.1 "Bacteriophage resistance mechanisms". Nature Reviews. Microbiology 8 (5): 317–27. May 2010. doi:10.1038/nrmicro2315. PMID 20348932.
- ↑ "Toxin-antitoxin systems in bacterial growth arrest and persistence". Nature Chemical Biology 12 (4): 208–14. April 2016. doi:10.1038/nchembio.2044. PMID 26991085.
- ↑ 8.0 8.1 "The decay of the chromosomally encoded ccdO157 toxin-antitoxin system in the Escherichia coli species". Genetics 181 (4): 1557–66. April 2009. doi:10.1534/genetics.108.095190. PMID 19189956.
- ↑ 9.0 9.1 "Horizontal gene transfer of chromosomal Type II toxin-antitoxin systems of Escherichia coli". FEMS Microbiology Letters 363 (3): fnv238. February 2016. doi:10.1093/femsle/fnv238. PMID 26667220.
- ↑ 10.0 10.1 10.2 "Toxins-antitoxins: plasmid maintenance, programmed cell death, and cell cycle arrest". Science 301 (5639): 1496–9. September 2003. doi:10.1126/science.1088157. PMID 12970556. Bibcode: 2003Sci...301.1496H.
- ↑ 11.0 11.1 "Comparative analysis of superintegrons: engineering extensive genetic diversity in the Vibrionaceae". Genome Research 13 (3): 428–42. March 2003. doi:10.1101/gr.617103. PMID 12618374.
- ↑ 12.0 12.1 12.2 12.3 "The art of selective killing: plasmid toxin/antitoxin systems and their technological applications". BioTechniques 45 (3): 344–6. September 2008. doi:10.2144/000112955. PMID 18778262.
- ↑ "Postsegregational killing does not increase plasmid stability but acts to mediate the exclusion of competing plasmids". Proceedings of the National Academy of Sciences of the United States of America 97 (23): 12643–8. November 2000. doi:10.1073/pnas.220077897. PMID 11058151. Bibcode: 2000PNAS...9712643C.
- ↑ 14.0 14.1 "Genetic addiction: selfish gene's strategy for symbiosis in the genome". Genetics 172 (2): 1309–23. February 2006. doi:10.1534/genetics.105.042895. PMID 16299387.
- ↑ "Hypothetical functions of toxin-antitoxin systems". Journal of Bacteriology 189 (17): 6089–92. September 2007. doi:10.1128/JB.00958-07. PMID 17616596.
- ↑ 16.0 16.1 "Toxin-antitoxin loci are highly abundant in free-living but lost from host-associated prokaryotes". Nucleic Acids Research 33 (3): 966–76. 2005. doi:10.1093/nar/gki201. PMID 15718296.
- ↑ 17.0 17.1 17.2 "RASTA-Bacteria: a web-based tool for identifying toxin-antitoxin loci in prokaryotes". Genome Biology 8 (8): R155. 2007. doi:10.1186/gb-2007-8-8-r155. PMID 17678530.
- ↑ "Chromosomal toxin-antitoxin loci can diminish large-scale genome reductions in the absence of selection". Molecular Microbiology 63 (6): 1588–605. March 2007. doi:10.1111/j.1365-2958.2007.05613.x. PMID 17367382.
- ↑ "An Escherichia coli chromosomal "addiction module" regulated by guanosine [corrected 3',5'-bispyrophosphate: a model for programmed bacterial cell death"]. Proceedings of the National Academy of Sciences of the United States of America 93 (12): 6059–63. June 1996. doi:10.1073/pnas.93.12.6059. PMID 8650219. Bibcode: 1996PNAS...93.6059A.
- ↑ "mazEF-mediated programmed cell death in bacteria: "what is this?"". Critical Reviews in Microbiology 41 (1): 89–100. February 2015. doi:10.3109/1040841X.2013.804030. PMID 23799870.
- ↑ 21.0 21.1 "What is the benefit to Escherichia coli of having multiple toxin-antitoxin systems in its genome?". Journal of Bacteriology 189 (17): 6101–8. September 2007. doi:10.1128/JB.00527-07. PMID 17513477.
- ↑ "Escherichia coli MazEF toxin-antitoxin system does not mediate programmed cell death". Journal of Basic Microbiology 56 (12): 1398–1402. December 2016. doi:10.1002/jobm.201600247. PMID 27259116.
- ↑ 23.0 23.1 23.2 23.3 "parD toxin-antitoxin system of plasmid R1--basic contributions, biotechnological applications and relationships with closely-related toxin-antitoxin systems". The FEBS Journal 277 (15): 3097–117. August 2010. doi:10.1111/j.1742-4658.2010.07722.x. PMID 20569269.
- ↑ "RelE, a global inhibitor of translation, is activated during nutritional stress". Proceedings of the National Academy of Sciences of the United States of America 98 (25): 14328–33. December 2001. doi:10.1073/pnas.251327898. PMID 11717402. Bibcode: 2001PNAS...9814328C.
- ↑ "Chromosomal toxin-antitoxin systems may act as antiaddiction modules". Journal of Bacteriology 190 (13): 4603–9. July 2008. doi:10.1128/JB.00357-08. PMID 18441063.
- ↑ "Novel toxins from type II toxin-antitoxin systems with acetyltransferase activity". Plasmid 93: 30–35. September 2017. doi:10.1016/j.plasmid.2017.08.005. PMID 28941941.
- ↑ 27.0 27.1 27.2 "The phage abortive infection system, ToxIN, functions as a protein-RNA toxin-antitoxin pair". Proceedings of the National Academy of Sciences of the United States of America 106 (3): 894–9. January 2009. doi:10.1073/pnas.0808832106. PMID 19124776. Bibcode: 2009PNAS..106..894F.
- ↑ 28.0 28.1 "AbiQ, an abortive infection mechanism from Lactococcus lactis". Applied and Environmental Microbiology 64 (12): 4748–56. December 1998. doi:10.1128/AEM.64.12.4748-4756.1998. PMID 9835558. Bibcode: 1998ApEnM..64.4748E.
- ↑ 29.0 29.1 "Escherichia coli mazEF-mediated cell death as a defense mechanism that inhibits the spread of phage P1". Molecular Genetics and Genomics 272 (2): 227–34. September 2004. doi:10.1007/s00438-004-1048-y. PMID 15316771.
- ↑ "Exclusion of T4 phage by the hok/sok killer locus from plasmid R1". Journal of Bacteriology 178 (7): 2044–50. April 1996. doi:10.1128/jb.178.7.2044-2050.1996. PMID 8606182.
- ↑ "A widespread bacteriophage abortive infection system functions through a Type IV toxin-antitoxin mechanism". Nucleic Acids Research 42 (7): 4590–605. April 2014. doi:10.1093/nar/gkt1419. PMID 24465005.
- ↑ "Battling Phages: How Bacteria Defend against Viral Attack". PLOS Pathogens 11 (6): e1004847. June 2015. doi:10.1371/journal.ppat.1004847. PMID 26066799.
- ↑ "Bacterial persistence: a model of survival in changing environments". Genetics 169 (4): 1807–14. April 2005. doi:10.1534/genetics.104.035352. PMID 15687275.
- ↑ "Molecular mechanisms underlying bacterial persisters". Cell 157 (3): 539–48. April 2014. doi:10.1016/j.cell.2014.02.050. PMID 24766804.
- ↑ "What Is the Link between Stringent Response, Endoribonuclease Encoding Type II Toxin-Antitoxin Systems and Persistence?" (in English). Frontiers in Microbiology 7: 1882. 2016. doi:10.3389/fmicb.2016.01882. PMID 27933045.
- ↑ "Prophages and Growth Dynamics Confound Experimental Results with Antibiotic-Tolerant Persister Cells". mBio 8 (6): e01964–17. December 2017. doi:10.1128/mBio.01964-17. PMID 29233898.
- ↑ "Reassessing the Role of Type II Toxin-Antitoxin Systems in Formation of Escherichia coli Type II Persister Cells". mBio 9 (3): e00640–18. June 2018. doi:10.1128/mBio.00640-18. PMID 29895634.
- ↑ "Endoribonuclease type II toxin-antitoxin systems: functional or selfish?". Microbiology 163 (7): 931–939. July 2017. doi:10.1099/mic.0.000487. PMID 28691660.
- ↑ 39.0 39.1 39.2 39.3 39.4 "Small toxic proteins and the antisense RNAs that repress them". Microbiology and Molecular Biology Reviews 72 (4): 579–89, Table of Contents. December 2008. doi:10.1128/MMBR.00025-08. PMID 19052321.
- ↑ "Large-Scale Analyses of Human Microbiomes Reveal Thousands of Small, Novel Genes". Cell 178 (5): 1245–1259.e14. August 2019. doi:10.1016/j.cell.2019.07.016. PMID 31402174.
- ↑ "The antisense RNA of the par locus of pAD1 regulates the expression of a 33-amino-acid toxic peptide by an unusual mechanism". Molecular Microbiology 37 (3): 652–60. August 2000. doi:10.1046/j.1365-2958.2000.02035.x. PMID 10931358.
- ↑ "The small RNA IstR inhibits synthesis of an SOS-induced toxic peptide". Current Biology 14 (24): 2271–6. December 2004. doi:10.1016/j.cub.2004.12.003. PMID 15620655.
- ↑ "Single transmembrane peptide DinQ modulates membrane-dependent activities". PLOS Genetics 9 (2): e1003260. February 7, 2013. doi:10.1371/journal.pgen.1003260. PMID 23408903.
- ↑ "Molecular characterization of long direct repeat (LDR) sequences expressing a stable mRNA encoding for a 35-amino-acid cell-killing peptide and a cis-encoded small antisense RNA in Escherichia coli". Molecular Microbiology 45 (2): 333–49. July 2002. doi:10.1046/j.1365-2958.2002.03042.x. PMID 12123448.
- ↑ "Nucleotide sequence and transcriptional analysis of a third function (Flm) involved in F-plasmid maintenance". Gene 66 (2): 259–68. June 1988. doi:10.1016/0378-1119(88)90362-9. PMID 3049248.
- ↑ "Repression of small toxic protein synthesis by the Sib and OhsC small RNAs". Molecular Microbiology 70 (5): 1076–93. December 2008. doi:10.1111/j.1365-2958.2008.06394.x. PMID 18710431. (Subscription content?)
- ↑ "Small untranslated RNA antitoxin in Bacillus subtilis". Journal of Bacteriology 187 (19): 6641–50. October 2005. doi:10.1128/JB.187.19.6641-6650.2005. PMID 16166525.
- ↑ "A novel family of plasmid-transferred anti-sense ncRNAs". RNA Biology 7 (2): 120–4. March 2010. doi:10.4161/rna.7.2.11184. PMID 20220307.
- ↑ "Salmonella Protein TimP Targets the Cytoplasmic Membrane and Is Repressed by the Small RNA TimR". mBio 11 (6): e01659–20, /mbio/11/6/mBio.01659–20.atom. November 2020. doi:10.1128/mBio.01659-20. PMID 33172998.
- ↑ "Mechanistic insights into type I toxin antitoxin systems in Helicobacter pylori: the importance of mRNA folding in controlling toxin expression". Nucleic Acids Research 45 (8): 4782–4795. May 2017. doi:10.1093/nar/gkw1343. PMID 28077560.
- ↑ "A cis-antisense RNA acts in trans in Staphylococcus aureus to control translation of a human cytolytic peptide". Nature Structural & Molecular Biology 19 (1): 105–12. December 2011. doi:10.1038/nsmb.2193. PMID 22198463. http://www.hal.inserm.fr/inserm-00696345/document.
- ↑ "Functional and structural insights of a Staphylococcus aureus apoptotic-like membrane peptide from a toxin-antitoxin module". The Journal of Biological Chemistry 287 (52): 43454–63. December 2012. doi:10.1074/jbc.M112.402693. PMID 23129767.
- ↑ "The vapBC operon from Mycobacterium smegmatis is an autoregulated toxin-antitoxin module that controls growth via inhibition of translation". Journal of Molecular Biology 390 (3): 353–67. July 2009. doi:10.1016/j.jmb.2009.05.006. PMID 19445953.
- ↑ "Mechanisms for Differential Protein Production in Toxin-Antitoxin Systems". Toxins 9 (7): 211. July 2017. doi:10.3390/toxins9070211. PMID 28677629.
- ↑ 55.0 55.1 "Cell killing by the F plasmid CcdB protein involves poisoning of DNA-topoisomerase II complexes". Journal of Molecular Biology 226 (3): 735–45. August 1992. doi:10.1016/0022-2836(92)90629-X. PMID 1324324.
- ↑ "MazF cleaves cellular mRNAs specifically at ACA to block protein synthesis in Escherichia coli". Molecular Cell 12 (4): 913–23. October 2003. doi:10.1016/s1097-2765(03)00402-7. PMID 14580342.
- ↑ "Global Analysis of the E. coli Toxin MazF Reveals Widespread Cleavage of mRNA and the Inhibition of rRNA Maturation and Ribosome Biogenesis". Molecular Cell 70 (5): 868–880.e10. June 2018. doi:10.1016/j.molcel.2018.04.026. PMID 29861158.
- ↑ "Toxin-mediated ribosome stalling reprograms the Mycobacterium tuberculosis proteome". Nature Communications 10 (1): 3035. July 2019. doi:10.1038/s41467-019-10869-8. PMID 31292443. Bibcode: 2019NatCo..10.3035B.
- ↑ "The Sole Mycobacterium smegmatis MazF Toxin Targets tRNALys to Impart Highly Selective, Codon-Dependent Proteome Reprogramming". Frontiers in Genetics 10: 1356. 2019. doi:10.3389/fgene.2019.01356. PMID 32117414.
- ↑ "Mycobacterial toxin MazF-mt6 inhibits translation through cleavage of 23S rRNA at the ribosomal A site". Proceedings of the National Academy of Sciences of the United States of America 110 (21): 8501–6. May 2013. doi:10.1073/pnas.1222031110. PMID 23650345. Bibcode: 2013PNAS..110.8501S.
- ↑ "Chapter 25 RNA Decay by Messenger RNA Interferases". RNA Turnover in Bacteria, Archaea and Organelles. Methods in Enzymology. 447. 2008. pp. 521–35. doi:10.1016/S0076-6879(08)02225-8. ISBN 978-0-12-374377-0.
- ↑ mRNA interferases, sequence-specific endoribonucleases from the toxin-antitoxin systems. Progress in Molecular Biology and Translational Science. 85. 2009. pp. 467–500. doi:10.1016/S0079-6603(08)00812-X. ISBN 978-0-12-374761-7.
- ↑ "Regulation of Type II Toxin-Antitoxin Systems: The Translation-Responsive Model" (in English). Frontiers in Microbiology 11: 895. 2020. doi:10.3389/fmicb.2020.00895. PMID 32431690.
- ↑ 64.0 64.1 "ε/ζ systems: their role in resistance, virulence, and their potential for antibiotic development". Journal of Molecular Medicine 89 (12): 1183–94. December 2011. doi:10.1007/s00109-011-0797-4. PMID 21822621.
- ↑ "New toxins homologous to ParE belonging to three-component toxin-antitoxin systems in Escherichia coli O157:H7". Molecular Microbiology 76 (3): 719–32. May 2010. doi:10.1111/j.1365-2958.2010.07129.x. PMID 20345661. https://hal.archives-ouvertes.fr/hal-00552629/file/PEER_stage2_10.1111%252Fj.1365-2958.2010.07129.x.pdf.
- ↑ "SecB-like chaperone controls a toxin-antitoxin stress-responsive system in Mycobacterium tuberculosis". Proceedings of the National Academy of Sciences of the United States of America 108 (20): 8438–43. May 2011. doi:10.1073/pnas.1101189108. PMID 21536872. Bibcode: 2011PNAS..108.8438B.
- ↑ "Interaction specificity, toxicity and regulation of a paralogous set of ParE/RelE-family toxin-antitoxin systems". Molecular Microbiology 77 (1): 236–51. July 2010. doi:10.1111/j.1365-2958.2010.07207.x. PMID 20487277.
- ↑ "An SOS-regulated type 2 toxin-antitoxin system". Journal of Bacteriology 191 (24): 7456–65. December 2009. doi:10.1128/JB.00963-09. PMID 19837801.
- ↑ "HicA of Escherichia coli defines a novel family of translation-independent mRNA interferases in bacteria and archaea". Journal of Bacteriology 191 (4): 1191–9. February 2009. doi:10.1128/JB.01013-08. PMID 19060138.
- ↑ "fMet". Nature Chemical Biology 13 (6): 640–646. June 2017. doi:10.1038/nchembio.2346. PMID 28369041. http://eprints.whiterose.ac.uk/118591/13/AtaT%20blocks%20translation%20initiation%20AAM.pdf.
- ↑ "Mutagenesis and functional characterization of the RNA and protein components of the toxIN abortive infection and toxin-antitoxin locus of Erwinia". Journal of Bacteriology 191 (19): 6029–39. October 2009. doi:10.1128/JB.00720-09. PMID 19633081.
- ↑ "A processed noncoding RNA regulates an altruistic bacterial antiviral system". Nature Structural & Molecular Biology 18 (2): 185–90. February 2011. doi:10.1038/nsmb.1981. PMID 21240270.
- ↑ "A novel family of Escherichia coli toxin-antitoxin gene pairs". Journal of Bacteriology 185 (22): 6600–8. November 2003. doi:10.1128/jb.185.22.6600-6608.2003. PMID 14594833.
- ↑ "The Toxin-Antitoxin System DarTG Catalyzes Reversible ADP-Ribosylation of DNA". Molecular Cell 64 (6): 1109–1116. December 2016. doi:10.1016/j.molcel.2016.11.014. PMID 27939941.
- ↑ "Molecular basis for DarT ADP-ribosylation of a DNA base". Nature 596 (7873): 597–602. August 2021. doi:10.1038/s41586-021-03825-4. PMID 34408320. Bibcode: 2021Natur.596..597S.
- ↑ Schuller, Marion; Raggiaschi, Roberto; Mikolcevic, Petra; Rack, Johannes G. M.; Ariza, Antonio; Zhang, YuGeng; Ledermann, Raphael; Tang, Christoph et al. (2023-07-06). "Molecular basis for the reversible ADP-ribosylation of guanosine bases" (in en). Molecular Cell 83 (13): 2303–2315.e6. doi:10.1016/j.molcel.2023.06.013. ISSN 1097-2765. PMID 37390817.
- ↑ "Type II toxin/antitoxin MqsR/MqsA controls type V toxin/antitoxin GhoT/GhoS". Environmental Microbiology 15 (6): 1734–44. June 2013. doi:10.1111/1462-2920.12063. PMID 23289863. Bibcode: 2013EnvMi..15.1734W.
- ↑ "A bacterial toxin inhibits DNA replication elongation through a direct interaction with the β sliding clamp". Molecular Cell 52 (5): 617–28. December 2013. doi:10.1016/j.molcel.2013.10.014. PMID 24239291.
- ↑ "Type VII Toxin/Antitoxin Classification System for Antitoxins that Enzymatically Neutralize Toxins". Trends in Microbiology 29 (5): 388–393. May 2021. doi:10.1016/j.tim.2020.12.001. PMID 33342606.
- ↑ Li, Ming; Gong, Luyao; Cheng, Feiyue; Yu, Haiying; Zhao, Dahe; Wang, Rui; Wang, Tian; Zhang, Shengjie et al. (2021-04-30). "Toxin-antitoxin RNA pairs safeguard CRISPR-Cas systems" (in en). Science 372 (6541): eabe5601. doi:10.1126/science.abe5601. ISSN 0036-8075. PMID 33926924. https://www.science.org/doi/10.1126/science.abe5601.
- ↑ "Temperature and growth rate effects on the hok/sok killer locus for enhanced plasmid stability". Biotechnology Progress 10 (6): 621–9. 1994. doi:10.1021/bp00030a600. PMID 7765697.
- ↑ "Combining the hok/sok, parDE, and pnd postsegregational killer loci to enhance plasmid stability". Applied and Environmental Microbiology 63 (5): 1917–24. May 1997. doi:10.1128/AEM.63.5.1917-1924.1997. PMID 9143123. Bibcode: 1997ApEnM..63.1917P.
- ↑ 83.0 83.1 "Prokaryotic toxin-antitoxin stress response loci". Nature Reviews. Microbiology 3 (5): 371–82. May 2005. doi:10.1038/nrmicro1147. PMID 15864262.
- ↑ 84.0 84.1 "Positive-selection vectors using the F plasmid ccdB killer gene". Gene 148 (1): 71–4. October 1994. doi:10.1016/0378-1119(94)90235-6. PMID 7926841.
- ↑ "A dual lethal system to enhance containment of recombinant micro-organisms". Microbiology 149 (Pt 12): 3595–601. December 2003. doi:10.1099/mic.0.26618-0. PMID 14663091.
External links
- RASTA – Rapid Automated Scan for Toxins and Antitoxins in Bacteria
 |