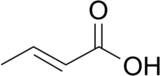
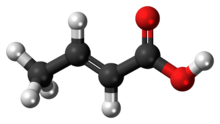
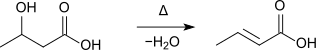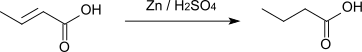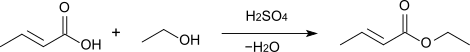Chemistry:Crotonic acid
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
(2E)-But-2-enoic acid | |
| Other names
(E)-But-2-enoic acid
(E)-2-Butenoic acid Crotonic acid trans-2-Butenoic acid β-Methylacrylic acid 3-Methylacrylic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C4H6O2 | |
| Molar mass | 86.090 g·mol−1 |
| Density | 1.02 g/cm3 |
| Melting point | 70 to 73 °C (158 to 163 °F; 343 to 346 K) |
| Boiling point | 185 to 189 °C (365 to 372 °F; 458 to 462 K) |
| Acidity (pKa) | 4.69 [1] |
| Hazards | |
| Safety data sheet | SIRI.org |
| Related compounds | |
Other anions
|
crotonate |
Related carboxylic acids
|
propionic acid acrylic acid butyric acid succinic acid malic acid tartaric acid fumaric acid pentanoic acid tetrolic acid |
Related compounds
|
butanol butyraldehyde crotonaldehyde 2-butanone |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Crotonic acid ((2E)-but-2-enoic acid) is a short-chain unsaturated carboxylic acid described by the formula CH3CH=CHCO2H. The name crotonic acid was given because it was erroneously thought to be a saponification product of croton oil.[2] It crystallizes as colorless needles from hot water. With a cis-alkene, Isocrotonic acid is an isomer of crotonic acid. Crotonic acid is soluble in water and many organic solvents. Its odor is similar to that of butyric acid.
Production
Crotonic acid produced industrially by oxidation of crotonaldehyde:[3][4]:230
- CH
3CH=CHCHO + 1/
2 O
2 → CH
3CH=CHCO
2H
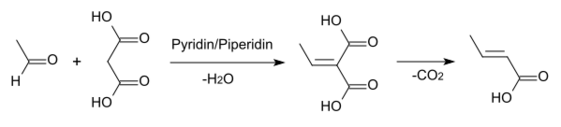
A number of other methods exist, including the Knoevenagel condensation of acetaldehyde with malonic acid in pyridine:[3]:229
The alkaline hydrolysis of allyl cyanide followed by the intramolecular rearrangement of the double bond:[5][6]
Furthermore, it is formed during the distillation of 3-hydroxybutyric acid:[7]
Properties
Crotonic acid crystallizes in the monoclinic crystal system in the space group P21/a (space group 14, position 3) with the lattice parameters a = 971 pm, b = 690 pm, c = 775 pm and β = 104.0°. The unit cell contains four formula units.[8]
Reactions
Crotonic acid converts into butyric acid by hydrogenation or by reduction with zinc and sulfuric acid.[9]
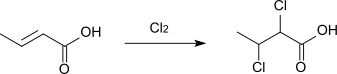
Upon treatment with chlorine or bromine, crotonic acid converts to 2,3-dihalobutyric acids:[9]
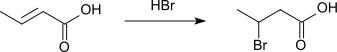
Crotonic acid adds hydrogen bromide to form 3-bromobutyric acid.[9][10]
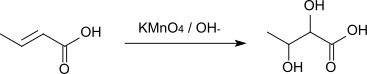
The reaction with alkaline potassium permanganate solution affords 2,3-dihydroxybutyric acid.[9]
Upon heating with acetic anhydride, crotonic acid converts to the acid anhydride:[11]
Esterification of crotonic acid using sulfuric acid as a catalyst provides the corresponding crotonate esters:
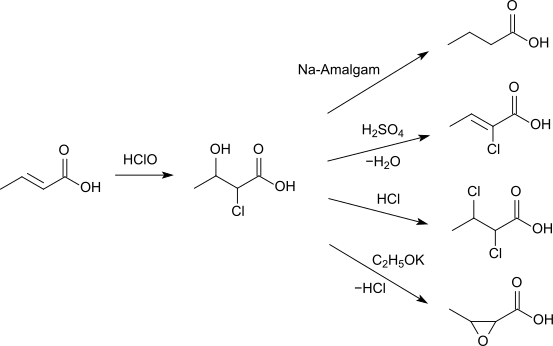
Crotonic acid reacts with hypochlorous acid to 2-chloro-3-hydroxybutyric acid. This can either be reduced with sodium amalgam to butyric acid, can form with sulfuric acid 2-chlorobutenoic acid, react with hydrogen chloride to 2,3-dichlorobutenoic acid or with potassium ethoxide to 3-methyloxirane-2-carboxylic acid.[12]
Crotonic acid reacts with ammonia at the alpha position in the presence of mercury(II) acetate. This reaction provides DL-threonine.[13]
Use
Crotonic acid is mainly used as a comonomer with vinyl acetate.[14] The resulting copolymers are used in paints and adhesives.[4]
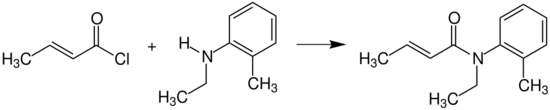
Crotonyl chloride reacts with N-ethyl-2-methylaniline (N-ethyl-o-toluidine) to provide crotamiton, which is used as an agent against scabies.[15]
Safety
Its -1">50 is 1 g/kg (oral, rats).[4] It irritates eyes, skin, and respiratory system.[14]
See also
References
- ↑ Dawson, R. M. C. (1959). Data for Biochemical Research. Oxford: Clarendon Press.
- ↑ Chisholm, Hugh, ed (1911). "Crotonic Acid". Encyclopædia Britannica. 7 (11th ed.). Cambridge University Press. p. 511.
- ↑ 3.0 3.1 Beyer, Hans; Walter, Wolfgang (1984) (in de). Organische Chemie. Stuttgart: S. Hirzel Verlag. ISBN 3-7776-0406-2.
- ↑ 4.0 4.1 4.2 Schulz, R. P.; Blumenstein, J.; Kohlpaintner, C. (2005). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a08_083.
- ↑ Rinne, A.; Tollens, B. (1871). "Ueber das Allylcyanür oder Crotonitril". Justus Liebigs Annalen der Chemie 159 (1): 105–109. doi:10.1002/jlac.18711590110. https://zenodo.org/record/1427303.
- ↑ Pomeranz, C. (1906). "Ueber Allylcyanid und Allylsenföl". Justus Liebigs Annalen der Chemie 351 (1–3): 354–362. doi:10.1002/jlac.19073510127. https://zenodo.org/record/1427569.
- ↑ Beilstein, F. (1893) (in de). Handbuch der organischen Chemie. 1 (3rd ed.). Verlag Leopold Voss. p. 506. http://www26.us.archive.org/stream/handbuchderorgan00beil#page/506/mode/2up.
- ↑ Shimizu, S.; Kekka, S.; Kashino, S.; Haisa, M. (1974). "Topochemical Studies. III. The Crystal and Molecular Structures of Crotonic Acid, CH3CH=CHCO2H, and Crotonamide, CH3CH=CHCONH2". Bulletin of the Chemical Society of Japan 47 (7): 1627–1631. doi:10.1246/bcsj.47.1627.
- ↑ 9.0 9.1 9.2 9.3 Heilbron (1953). "Crotonic acid". Dictionary of Organic Compounds 1: 615. https://archive.org/stream/dictionaryoforga011095mbp#page/n633/mode/2up.
- ↑ Lovén, J. M.; Johansson, H. (1915). "Einige schwefelhaltige β-Substitutionsderivate der Buttersäure". Berichte der deutschen chemischen Gesellschaft 48 (2): 1254–1262. doi:10.1002/cber.19150480205. https://zenodo.org/record/1426589.
- ↑ Clover, A. M.; Richmond, G. F. (1903). "The Hydrolysis of Organic Peroxides and Peracids". American Chemical Journal 29 (3): 179–203. https://archive.org/stream/americanchemical291903balt#page/178/mode/2up.
- ↑ Beilstein, F. (1893) (in de). Handbuch der organischen Chemie. 1 (3rd ed.). Verlag Leopold Voss. p. 562. http://www26.us.archive.org/stream/handbuchderorgan00beil#page/562/mode/2up.
- ↑ Carter, H. E.; West, H. D. (1955). "dl-Threonine". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=cv3p0813.; Collective Volume, 3, pp. 813
- ↑ 14.0 14.1 Entry on Butensäuren. at: Römpp Online. Georg Thieme Verlag, retrieved January 7, 2020.
- ↑ Kleemann, A.; Engel, J.. Pharmazeutische Wirkstoffe: Synthesen, Patente, Anwendungen. 5 (2nd rev. and updated ed.). Stuttgart & New York: Georg Thieme Verlag. p. 251. ISBN 3-13-558402-X.
 |