Biology:Erythrocruorin
| Globin, extracellular | |
|---|---|
 | |
| Identifiers | |
| Symbol | Haemoglobin_extracell |
| InterPro | IPR014610 |
| Annelid erythrocruorin linker subunit, C-terminal | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | Eryth_link_C | ||||||||
| Pfam | PF16915 | ||||||||
| InterPro | IPR031639 | ||||||||
| CATH | 2gtlM02 | ||||||||
| SCOP2 | d2gtlm1 / SCOPe / SUPFAM | ||||||||
| CDD | cd11673 | ||||||||
| |||||||||
Erythrocruorin (from Greek eruthros "red" + Latin cruor "blood"), and the similar chlorocruorin (from Greek khlōros "green" + Latin cruor "blood"), are large oxygen-carrying hemeprotein complexes, which have a molecular mass greater than 3.5 million daltons.[1] Both are sometimes called giant hemoglobin or hexagonal bilayer haemoglobin. They are found in many annelids and arthropods (including some insects).[2]
Chlorocruorin is particularly found in certain marine polychaetes.[3][4][5]
Structure
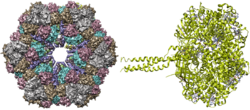
Two structures of erythrocruorin have been resolved. The protein is a highly symmetric assembly made from heme-binding globins and unique linker proteins.[1][6]
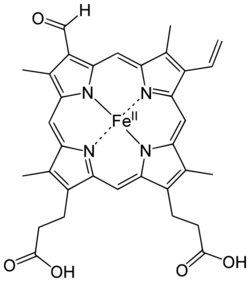
The only significant difference between chlorocruorin and erythrocruorin is that chlorocruorin carries an abnormal heme group structure. Both contain many 16–17 kDa myoglobin-like subunits arranged in a giant complex of over a hundred subunits with interlinking proteins as well with a total weight exceeding 3600 kDa. [6]
Giant hemoglobin is composed of multiple heme-containing globin chains and linker (InterPro: IPR031639) chains. Each species have different amounts of genes for these chains. For example, while a Lamellibrachia sp. has four kinds of globin chains and two kinds of linker chains, Sabella spallanzanii has three globin chains and three linker chains.[6] The exact stoichiometric ratios and arrangement is unknown, but is thought to resemble that of erythocrorins.
Properties
Erythrocruorin has a weaker affinity for oxygen than that of most hemoglobins. A dichromatic compound, chlorocruorin is noted for appearing green in dilute solutions, though it appears light red when found in concentrated solutions.[7][8][9]
This enormous macromolecule is typically found free floating in the plasma, and not contained within red blood cells.[6][10]
References
- ↑ 1.0 1.1 "Structural hierarchy in erythrocruorin, the giant respiratory assemblage of annelids". Proceedings of the National Academy of Sciences of the United States of America 97 (13): 7107–11. June 2000. doi:10.1073/pnas.97.13.7107. PMID 10860978. Bibcode: 2000PNAS...97.7107R.
- ↑ "The structure of the giant haemoglobin from Glossoscolex paulistus". Acta Crystallographica. Section D, Biological Crystallography 71 (Pt 6): 1257–71. June 2015. doi:10.1107/S1399004715005453. PMID 26057666.
- ↑ H. Munro Fox (1 April 1933). "The Blood Circulation of Animals Possessing Chlorocruorin". Proceedings of the Royal Society B 112 (779): 479–495. doi:10.1098/rspb.1938.0042.
- ↑ R. F. Ewer; H. Munro Fox (9 August 1940). "On the Function of Chlorocruorin". Proceedings of the Royal Society B 129 (855): 137–153. doi:10.1098/rspb.1940.0033. Bibcode: 1940RSPSB.129..137E.
- ↑ D.W. Ewer (1941). "The blood systems of Sabella and Spirographis". Quarterly Journal of Microscopical Science 82 (s2): 587–619. http://jcs.biologists.org/cgi/reprint/s2-82/328/587. Retrieved 1 May 2010.
- ↑ 6.0 6.1 6.2 6.3 "The primary structure of globin and linker chains from the chlorocruorin of the polychaete Sabella spallanzanii". The Journal of Biological Chemistry 276 (28): 26384–90. July 2001. doi:10.1074/jbc.M006939200. PMID 11294828.
- ↑ H. Munro Fox (1 February 1926). "Chlorocruorin: A Pigment Allied to Haemoglobin". Proceedings of the Royal Society B 99 (696): 199–220. doi:10.1098/rspb.1926.0008.
- ↑ H. Munro Fox (1 September 1932). "The Oxygen Affinity of Chlorocruorin". Proceedings of the Royal Society B 111 (772): 356–363. doi:10.1098/rspb.1932.0060.
- ↑ H. Munro Fox (19 October 1949). "On Chlorocruorin and Haemoglobin". Proceedings of the Royal Society B 136 (884): 378–388. doi:10.1098/rspb.1949.0031. PMID 18143368. Bibcode: 1949RSPSB.136..378F.
- ↑ "Giant Hexagonal Bilayer Hemoglobins". Chem Rev 96 (8): 3113–3124. 19 December 1996. doi:10.1021/cr9600058. PMID 11848854.
External links
- Erythrocruorins at the US National Library of Medicine Medical Subject Headings (MeSH)
- chlorocruorin at the US National Library of Medicine Medical Subject Headings (MeSH)
- RSDB Molecule of the Month: 159 Erythrocruorin
 |