Chemistry:Sodium zirconium cyclosilicate
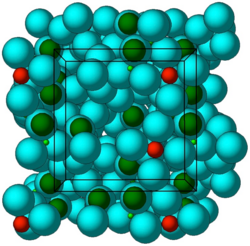 Crystal structure of ZS-9. Blue spheres = oxygen atoms, red spheres = zirconium atoms, green spheres = silicon atoms. | |
| Clinical data | |
|---|---|
| Trade names | Lokelma |
| Other names | ZS-9 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a618035 |
| License data | |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Not absorbed |
| Excretion | Feces |
| Identifiers | |
| |
| CAS Number | |
| DrugBank | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | (2Na·H2O·3H4SiO4·H4ZrO6)n |
Sodium zirconium cyclosilicate, sold under the brand name Lokelma, is a medication used to treat high blood potassium.[4] Onset of effects occurs in one to six hours.[4] It is taken by mouth.[4]
Common side effects include swelling and low blood potassium.[4] Use is likely safe in pregnancy and breastfeeding.[4] It works by binding potassium ions in the gastrointestinal tract which is then lost in the stool.[4][5]
Sodium zirconium cyclosilicate was approved for medical use in the European Union and in the United States in 2018.[4][3][6] It was developed by AstraZeneca.[4]
Medical use
Sodium zirconium cyclosilicate is used to treat high blood potassium.[4] Onset of effects occurs in one to six hours.[4]
Mechanism of action
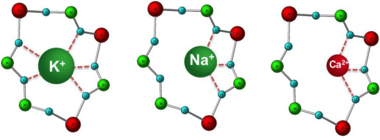
ZS-9 is a zirconium silicate. Zirconium silicates have been extensively used in medical and dental applications because of their proven safety.[7] 11 zirconium silicates were screened by an iterative optimization process. ZS-9 selectively captures potassium ions, presumably by mimicking the actions of physiologic potassium channels.[8] ZS-9 is an inorganic cation exchanger crystalline with a high capacity to entrap monovalent cations, specifically potassium and ammonium ions, in the GI tract.
Background
Hyperkalemia is rare among those who are otherwise healthy.[9] Among those who are in hospital, rates are between 1% and 2.5%.[10] Common causes include kidney failure, hypoaldosteronism, and rhabdomyolysis.[11] A number of medications can also cause high blood potassium including spironolactone, NSAIDs, and angiotensin converting enzyme inhibitors.[11]
There is no universally accepted definition of what level of hyperkalemia is mild, moderate, or severe.[12] However, if hyperkalemia causes any ECG change it is considered a medical emergency[12] due to a risk of potentially fatal abnormal heart rhythms and is treated urgently.[12] Potassium levels greater than 6.5 to 7.0 mmol/L in the absence of ECG changes are managed aggressively.[12] Several approaches are used to treat hyperkalemia.[12] Other approved potassium binders in the United States include patiromer and sodium polystyrene sulfonate.[13]
Hyperkalemia, particularly if severe, is a marker for an increased risk of death.[14] However, there is disagreement regarding whether a modestly elevated levels directly causes problems. One viewpoint is that mild to moderate hyperkalemia is a secondary effect that denotes underlying medical problems.[14] Accordingly, these problems are both proximate and ultimate causes of death,[14]
History
In the United States, regulatory approval of ZS-9 was rejected by the Food and Drug Administration (FDA) in May 2016, due to issues associated with manufacturing.[15] On 18 May 2018, the FDA approved sodium zirconium cyclosilicate for treatment of adults with hyperkalemia.[16]
It was first practically synthesized by UOP in the late 1990s. (reference -zirconium silicate and zirconium germate molecular sieves and the process of using the same, US Patent 5,888,472) the recognition of the unique ion exchange properties and the potential use to remove toxins from the body were identified shortly thereafter ("process for removing toxins from bodily fluids using zirconium or titanium microporous compositions, US Patent 6,332,985).
Research
One review found a decrease in potassium of 0.17 mEq/L at one hour and 0.67 mEq/L at 48 hours.[17]
It appears effective in people with chronic kidney disease, diabetes, and heart failure.[5] Use has been studied for up to a year.[5]
References
- ↑ "Summary Basis of Decision (SBD) for Lokelma". 23 October 2014. https://hpr-rps.hres.ca/reg-content/summary-basis-decision-detailTwo.php?linkID=SBD00468&lang=en.
- ↑ "Lokelma- sodium zirconium cyclosilicate powder, for suspension". 30 September 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=90bf8e28-748d-4e4b-a19f-9cf483370eff.
- ↑ 3.0 3.1 "Lokelma EPAR". 17 September 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/lokelma.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 4.9 "Sodium Zirconium Cyclosilicate Monograph for Professionals". https://www.drugs.com/monograph/sodium-zirconium-cyclosilicate.html.
- ↑ 5.0 5.1 5.2 "Sodium Zirconium Cyclosilicate: A Review in Hyperkalaemia". Drugs 78 (15): 1605–1613. October 2018. doi:10.1007/s40265-018-0991-6. PMID 30306338.
- ↑ "Drug Approval Package: Lokelma (sodium zirconium cyclosilicate)". 8 June 2018. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/207078Orig1s000TOC.cfm.
- ↑ Denry I, Kelly JR. State of the art of zirconia for dental applications. Dental Materials. Volume 24, Issue 3, March 2008, Pages 299–307
- ↑ "Characterization of structure and function of ZS-9, a K+ selective ion trap". PLOS ONE 9 (12): e114686. 2014. doi:10.1371/journal.pone.0114686. PMID 25531770. Bibcode: 2014PLoSO...9k4686S.
- ↑ "Updates in hyperkalemia: Outcomes and therapeutic strategies". Reviews in Endocrine & Metabolic Disorders 18 (1): 41–47. March 2017. doi:10.1007/s11154-016-9384-x. PMID 27600582.
- ↑ "Investigating hyperkalaemia in adults". BMJ 351: h4762. October 2015. doi:10.1136/bmj.h4762. PMID 26487322.
- ↑ 11.0 11.1 "Pathogenesis, diagnosis and management of hyperkalemia". Pediatric Nephrology 26 (3): 377–84. March 2011. doi:10.1007/s00467-010-1699-3. PMID 21181208.
- ↑ 12.0 12.1 12.2 12.3 12.4 Brenner and Rector's The Kidney (Chapter 17, page 672, 9th ed.). Elsevier. 2012. ISBN 978-1-4160-6193-9.
- ↑ "Damned if you do, damned if you don't: potassium binding resins in hyperkalemia". Clinical Journal of the American Society of Nephrology 5 (10): 1723–6. October 2010. doi:10.2215/CJN.03700410. PMID 20798253.
- ↑ 14.0 14.1 14.2 "Management of patients with acute hyperkalemia". CMAJ 182 (15): 1631–5. October 2010. doi:10.1503/cmaj.100461. PMID 20855477.
- ↑ Ben Adams (27 May 2016). "AstraZeneca's $2.7B hyperkalemia drug ZS-9 rejected by FDA". FierceBiotech. http://www.fiercebiotech.com/biotech/astrazeneca-s-2-7b-hyperkalemia-drug-zs-9-rejected-by-fda.
- ↑ "Lokelma (Sodium zirconium cyclosilicate) FDA Approval History". https://www.drugs.com/history/lokelma.html.
- ↑ "Systematic Review and Meta-Analysis of Patiromer and Sodium Zirconium Cyclosilicate: A New Armamentarium for the Treatment of Hyperkalemia". Pharmacotherapy 37 (4): 401–411. April 2017. doi:10.1002/phar.1906. PMID 28122118.
Further reading
External links
- "Sodium zirconium cyclosilicate". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/sodium%20zirconium%20cyclosilicate.
 |

