Chemistry:Omeprazole
Omeprazole, sold under the brand names Prilosec and Losec among others, is a medication used in the treatment of gastroesophageal reflux disease (GERD), peptic ulcer disease, and Zollinger–Ellison syndrome.[1] It is also used to prevent upper gastrointestinal bleeding in people who are at high risk.[1] Omeprazole is a proton-pump inhibitor (PPI) and its effectiveness is similar to that of other PPIs.[2] It can be taken by mouth or by injection into a vein.[1][3] It is also available in the fixed-dose combination medication omeprazole/sodium bicarbonate as Zegerid[4][5] and as Konvomep.[6]
Common side effects include nausea, vomiting, headaches, abdominal pain, and increased intestinal gas.[1][7] Serious side effects may include Clostridioides difficile colitis, an increased risk of pneumonia, an increased risk of bone fractures, and the potential of masking stomach cancer.[1] Whether it is safe for use in pregnancy is unclear.[1] It works by blocking the release of stomach acid.[1]
Omeprazole was patented in 1978 and approved for medical use in 1988.[8][9][10] It is on the World Health Organization's List of Essential Medicines.[11] It is available as a generic medication.[1] In 2023, it was the tenth most commonly prescribed medication in the United States, with more than 45 million prescriptions.[12][13] It is also available without a prescription in the United States.[14][15]
Medical uses
Omeprazole can be used in the treatment of gastroesophageal reflux disease (GERD), heartburn, peptic ulcers, erosive esophagitis, Zollinger–Ellison syndrome, and eosinophilic esophagitis.[16][1]
Peptic ulcers
Peptic ulcers may be treated with omeprazole. Infection with Helicobacter pylori can be treated by taking omeprazole, amoxicillin, and clarithromycin together for 7–14 days.[17] Amoxicillin may be replaced with metronidazole in patients who are allergic to penicillin.[18]
Adverse effects
Adverse effects occurring in at least 1% of people include:[19]
- Central nervous system: headache (7%), dizziness (2%)
- Respiratory: upper respiratory tract infection (2%), cough (1%)
- Gastrointestinal: abdominal pain (5%), diarrhea (4%), nausea (4%), vomiting (3%), flatulence (3%), acid regurgitation (2%), constipation (2%)
- Neuromuscular and skeletal: back pain (1%), weakness (1%)
- Dermatologic: rash (2%)
Other concerns related to adverse effects are:
- Recurrence of Clostridioides difficile associated diarrhea[20]
- Osteoporosis-related fractures[21][22]
- Hypomagnesemia[23]
Concern has been expressed regarding vitamin B12[24] and iron malabsorption,[25] but effects seem to be insignificant, especially when supplement therapy is provided.[26]
Since their introduction, proton-pump inhibitors (PPIs, especially omeprazole) have also been associated with several cases of acute interstitial nephritis,[27] an inflammation of the kidneys that often occurs as an adverse drug reaction.
Long-term use
Long-term use of PPIs is strongly associated with the development of benign polyps from fundic glands (which is distinct from fundic gland polyposis); these polyps do not cause cancer and resolve when PPIs are discontinued. No association is seen between PPI use and cancer, but use of PPIs may mask gastric cancers or other serious gastric problems.[28]
There is a possible association between long-term use and dementia which requires further study to confirm.[29]
An article published in 2013 claims that the long-term use of PPIs is associated with decreased calcium absorption (causing increased risk of osteoporosis and fractures), decreased magnesium absorption (causing electrolyte disturbances), and increased risk of certain infections, such as C. difficile and community-acquired pneumonia. The authors hypothesize that this is due to decreased stomach acid production.[30]
Pregnancy and breastfeeding
The safety of using omeprazole has not been established in pregnant or breastfeeding women.[7] Epidemiological data do not show an increased risk of major birth defects after maternal use of omeprazole during pregnancy.[31]
Interactions

Important drug interactions are rare.[32][33] However, the most significant major drug interaction concern is the decreased activation of clopidogrel when taken together with omeprazole.[34] Although still controversial,[35] this may increase the risk of stroke or heart attack in people taking clopidogrel to prevent these events.
This interaction is possible because omeprazole is an inhibitor of the enzymes CYP2C19 and CYP3A4.[36] Clopidogrel is an inactive prodrug that partially depends on CYP2C19 for conversion to its active form. Inhibition of CYP2C19 may block the activation of clopidogrel, which could reduce its effects.[37][38]
Almost all benzodiazepines are metabolised by the CYP3A4 and CYP2D6 pathways, and inhibition of these enzymes results in a higher area under the curve (i.e., the total effect over time of a given dose). Other examples of drugs dependent on CYP3A4 for their metabolism are escitalopram,[39] warfarin,[40] oxycodone, tramadol, and oxymorphone. The concentrations of these drugs may increase if they are used concomitantly with omeprazole.[41]
Omeprazole is also a competitive inhibitor of p-glycoprotein, as are other PPIs.[42]
Drugs that depend on an acidic stomach environment (such as ketoconazole or atazanavir) may be poorly absorbed, whereas acid-labile antibiotics (such as erythromycin which is a very strong CYP3A4 inhibitor) may be absorbed to a greater extent than normal due to the more alkaline environment of the stomach.[41]
St. John's wort (Hypericum perforatum) and Ginkgo biloba significantly reduce plasma concentrations of omeprazole through induction of CYP3A4 and CYP2C19.[43]
Pharmacology
Omeprazole irreversibly blocks the enzyme system on parietal cells that is needed for the secretion of gastric acid. It is a specific H+/K+ATPase inhibitor. This is the enzyme needed for the final step in the secretion of gastric acid.[44]
Pharmacokinetics
The absorption of omeprazole takes place in the small intestine and is usually completed within three to six hours. The systemic bioavailability of omeprazole after repeated doses is about 60%.[45] Omeprazole has a volume of distribution of 0.4 L/kg. It has high plasma protein binding of 95%.[46]
Omeprazole is completely metabolized by the cytochrome P450 system, mainly in the liver, by CYP2C19 and CYP3A4 isoenzymes.[7] Identified metabolites are the sulfone, the sulfide, and hydroxy-omeprazole. About 77% of an orally given dose is excreted as metabolites in the urine, and the remainder is found in the feces, primarily originating from bile secretion.[47] Omeprazole has a half life of 0.5 to 1 hour.[47]
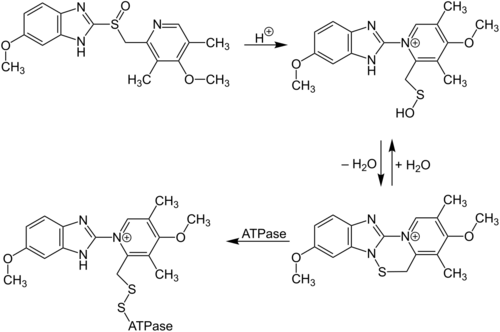
Bioactivation
As with all structually-similar benzimidazole proton pump inhibitors, omeprazole is a prodrug. A basic molecule, it accumulates in the acidic canaliculi of parietal cells in a protonated form where the S=O group becomes S-OH, which in turn is interconvertible with an achiral, reactive sulfenamide form. The sulfonamide form is able to attach onto the cysteine residue on the H+/K+-ATPase, thereby irreversibly inhibiting it.[48]
Chirality
The two different chiralities of omeprazole are both metabolized into inactive products by cytochrome P450 enzymes, but each chirality is differently inactivated by specific isozymes. Compared to the (R)-enantiomer, the (S)-enantiomer is relatively more resistant to metabolism, especially metabolism by CYP2C19[49] (if it's processed by CYP2C19 at all).[50] As a result, among people with a more active version of CYP2C19 ("extensive metabolizers"), the (R) half of a dose of omeprazole is likely to perform more poorly. Conversely, among those with a less active version of CYP2C19 ("poor metabolizers"), more the (R) half is expected to survive metabolism and end up useful. The proportion of the poor metabolizer phenotype varies widely between populations, from 2.0 to 2.5% in African Americans and white Americans to >20% in Asians. Several pharmacogenomics studies have suggested that PPI treatment should be tailored according to CYP2C19 metabolism status.[51]
AstraZeneca also developed esomeprazole (Nexium) which is a eutomer, purely the (S)-enantiomer, rather than a racemate like omeprazole.[52]
Mechanism of action
Omeprazole is a selective and irreversible proton pump inhibitor. It suppresses stomach acid secretion by specific inhibition of the H+/K+-ATPase system found at the secretory surface of gastric parietal cells. Because this enzyme system is regarded as the acid (proton, or H+) pump within the gastric mucosa, omeprazole inhibits the final step of acid production.[44]
Omeprazole also inhibits both basal and stimulated acid secretion irrespective of the stimulus[47] as it blocks the last step in acid secretion.[47] The drug binds non-competitively so it has a dose-dependent effect.[46]
The inhibitory effect of omeprazole occurs within one hour after oral administration. The maximum effect occurs within two hours. The duration of inhibition is up to 72 hours. When omeprazole is stopped, baseline stomach acid secretory activity returns after three to five days. The inhibitory effect of omeprazole on acid secretion will plateau after four days of repeated daily dosing.[53]
Omeprazole is only effective on active H+/K+-ATPase pumps. These pumps are stimulated in the presence of food to aid in digestion.[54]

Chemistry
Omeprazole contains a tricoordinated sulfinyl sulfur in a pyramidal structure and therefore can exist as either the (S)- or (R)-enantiomers. Omeprazole is a racemate, an equal mixture of the two.[48]
Measurement in body fluids
Omeprazole may be quantified in plasma or serum to monitor therapy or to confirm a diagnosis of poisoning in hospitalized patients. Plasma omeprazole concentrations are usually in a range of 0.2–1.2 mg/L in persons receiving the drug therapeutically by the oral route and 1–6 mg/L in people with acute overdose. Enantiomeric chromatographic methods are available to distinguish esomeprazole from racemic omeprazole.[55]
History
Omeprazole was first made in 1979 by Swedish AB Hässle, part of Astra AB. It was the first of the proton pump inhibitors (PPI).[56][57] Astra AB, now AstraZeneca, launched it as an ulcer medicine under the name Losec in Sweden. It was first sold in the United States in 1989 under the brand name Losec. In 1990, at the request of the US Food and Drug Administration, the brand name Losec was changed to Prilosec to avoid confusion with the diuretic Lasix (furosemide).[58] The new name led to confusion between omeprazole (Prilosec) and fluoxetine (Prozac), an antidepressant.[58] Prilosec is owned by Procter & Gamble in alliance with AstraZeneca[59] and the product is designed to address frequent heartburn, which can be triggered by various factors such as certain foods, stress, and smoking.
Prilosec was first introduced in 1989 as a prescription medication approved by the FDA for the treatment of severe heartburn.[60] In 2003, Prilosec OTC was launched as the first over-the-counter option for managing frequent heartburn.[61] It is known for its advertising campaign featuring Larry the Cable Guy as the spokesperson for the brand, during the 2010s, emphasizing the concept of "Zero Heartburn".[62]
Society and culture
Economics
When Prilosec's US patent expired in April 2001, AstraZeneca introduced esomeprazole (Nexium) as a patented replacement drug.[63] Many companies introduced generics as AstraZeneca's patents expired worldwide, which are available under many brand names.
Omeprazole was a subject of a patent litigation in the U.S.[64] The invention involved application of two different coatings to a drug in pill form to ensure that the omeprazole did not disintegrate before reaching its intended site of action in stomach. Although the solution by means of two coatings was obvious, the patent was found valid, because the source of the problem was non-obvious and was discovered by the patentee.[65]
In September 2023, AstraZeneca announced it would pay $425 million to settle product liability litigations against Prilosec in the United States.[66]
Brand names
Brand names include Losec, Prilosec, Zegerid, Miracid, and Omez.[67][1]
Veterinary uses
In February 2025, the Committee for Veterinary Medicinal Products of the European Medicines Agency adopted a positive opinion, recommending the granting of a marketing authorization for the veterinary medicinal product Omeprazole TriviumVet, gastro-resistant capsule, hard, intended for dogs.[68] The applicant for this veterinary medicinal product is TriviumVet DAC.[68] Omeprazole TriviumVet was authorized for veterinary use in the European Union in April 2025.[69]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 "Omeprazole". The American Society of Health-System Pharmacists. https://www.drugs.com/monograph/omeprazole.html.
- ↑ "[99 Comparative effectiveness of proton pump inhibitors | Therapeutics Initiative"]. 28 June 2016. http://www.ti.ubc.ca/2016/06/28/99-comparative-effectiveness-proton-pump-inhibitors/.
- ↑ "Omeprazole 40 mg Powder for Solution for Infusion". 10 February 2016. https://www.medicines.org.uk/emc/medicine/25259.
- ↑ "Zegerid- omeprazole and sodium bicarbonate powder, for suspension Zegerid- omeprazole and sodium bicarbonate capsule". 4 March 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=cd6868b9-5824-442b-8d65-4db29ecb70a4.
- ↑ "Zegerid OTC- omeprazole and sodium bicarbonate capsule, gelatin coated". 5 December 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5ad1ca2b-3119-b8c2-e053-2a91aa0a2f77.
- ↑ "Konvomep- omeprazole and sodium bicarbonate kit". 30 August 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=688ed6f1-d78a-4006-a682-57021cb38a3e.
- ↑ 7.0 7.1 7.2 Davis's Drug Guide for Nurses (14th ed.). F.A. Davis Company. 2015. pp. 924–925. ISBN 978-0-8036-4085-6. OCLC 881473728.
- ↑ Lagercrantz, Samuel (29 November 2022). "The stomach medication that became the biggest blockbuster of the 1990s". https://www.lifesciencesweden.se/article/view/883566/the_stomach_medication_that_became_the_biggest_blockbuster_of_the_1990s.
- ↑ "Product Details for NDA 019810". 5 October 1995. https://www.accessdata.fda.gov/scripts/cder/ob/results_product.cfm?Appl_Type=N&Appl_No=019810#3275.
- ↑ Analogue-based Drug Discovery. John Wiley & Sons. 2006. p. 445. ISBN 9783527607495. https://books.google.com/books?id=FjKfqkaKkAAC&pg=PA445. Retrieved 29 June 2020.
- ↑ The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. 2023. WHO/MHP/HPS/EML/2023.02.
- ↑ "The Top 300 of 2023". https://clincalc.com/DrugStats/Top300Drugs.aspx.
- ↑ "Omeprazole Drug Usage Statistics, United States, 2014 - 2023". https://clincalc.com/DrugStats/Drugs/Omeprazole.
- ↑ "Questions and Answers on Prilosec OTC (omeprazole)". 3 November 2018. https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/questions-and-answers-prilosec-otc-omeprazole.
- ↑ "Drug Approval Package: Prilosec (Omeprazole Magnesium) NDA #021229". https://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/21-229_Prilosec.cfm.
- ↑ "Proton pump inhibitors for eosinophilic oesophagitis". Current Opinion in Gastroenterology 29 (4): 416–420. July 2013. doi:10.1097/MOG.0b013e32835fb50e. PMID 23449027.
- ↑ "Meta-analysis: duration of first-line proton-pump inhibitor based triple therapy for Helicobacter pylori eradication". Annals of Internal Medicine 147 (8): 553–562. October 2007. doi:10.7326/0003-4819-147-8-200710160-00008. PMID 17938394.
- ↑ "Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report". Gut 56 (6): 772–781. June 2007. doi:10.1136/gut.2006.101634. PMID 17170018.
- ↑ "Omeprazole. An updated review of its pharmacology and therapeutic use in acid-related disorders". Drugs 42 (1): 138–170. July 1991. doi:10.2165/00003495-199142010-00008. PMID 1718683.
- ↑ "Risk factors for recurrence, complications and mortality in Clostridium difficile infection: a systematic review". PLOS ONE 9 (6). June 2014. doi:10.1371/journal.pone.0098400. PMID 24897375. Bibcode: 2014PLoSO...998400A.
- ↑ "Long-term proton pump inhibitor therapy and risk of hip fracture". JAMA 296 (24): 2947–2953. December 2006. doi:10.1001/jama.296.24.2947. PMID 17190895.
- ↑ "Proton pump inhibitors and risk of fractures: a meta-analysis of 11 international studies". The American Journal of Medicine 124 (6): 519–526. June 2011. doi:10.1016/j.amjmed.2011.01.007. PMID 21605729.
- ↑ "Systematic review: hypomagnesaemia induced by proton pump inhibition". Alimentary Pharmacology & Therapeutics 36 (5): 405–413. September 2012. doi:10.1111/j.1365-2036.2012.05201.x. PMID 22762246.
- ↑ "Potential gastrointestinal effects of long-term acid suppression with proton pump inhibitors". Alimentary Pharmacology & Therapeutics 15 (7): 1085–1086. July 2001. doi:10.1046/j.1365-2036.2001.0994a.x. PMID 11421886.
- ↑ "Association between proton pump inhibitor use and anemia: a retrospective cohort study". Digestive Diseases and Sciences 56 (8): 2349–2353. August 2011. doi:10.1007/s10620-011-1589-y. PMID 21318590.
- ↑ "Effect of proton pump inhibitors on vitamins and iron". The American Journal of Gastroenterology 104 (Suppl 2): S5–S9. March 2009. doi:10.1038/ajg.2009.45. PMID 19262546.
- ↑ "Proton pump inhibitor-induced acute interstitial nephritis". British Journal of Clinical Pharmacology 64 (6): 819–823. December 2007. doi:10.1111/j.1365-2125.2007.02927.x. PMID 17635502.
- ↑ "Proton pump inhibitor therapy and potential long-term harm". Current Opinion in Endocrinology, Diabetes, and Obesity 21 (1): 3–8. February 2014. doi:10.1097/med.0000000000000031. PMID 24310148.
- ↑ "Proton pump inhibitors: Risks of long-term use". Journal of Gastroenterology and Hepatology 32 (7): 1295–1302. July 2017. doi:10.1111/jgh.13737. PMID 28092694.
- ↑ "Long-Term Consequences of Chronic Proton Pump Inhibitor Use". US Pharmacist 38 (12): 38–42. December 2013. https://www.uspharmacist.com/article/longterm-consequences-of-chronic-proton-pump-inhibitor-use. Retrieved 28 December 2020.
- ↑ "Use of proton-pump inhibitors in early pregnancy and the risk of birth defects". The New England Journal of Medicine 363 (22): 2114–2123. November 2010. doi:10.1056/NEJMoa1002689. PMID 21105793.
- ↑ "2014 Treatments for Acid-Peptic Diseases.". University of Minnesota Medical School Duluth. http://www.d.umn.edu/~jfitzake/Lectures/DMED/Antiulcer/Treatment/ReducePain/PPIs/PPIsSE.html.
- ↑ "Proton Pump Inhibitor: Use in Adults". CMS Medicaid Integrity Program. http://www.cms.gov/Medicare-Medicaid-Coordination/Fraud-Prevention/Medicaid-Integrity-Education/Pharmacy-Education-Materials/Downloads/ppi-adult-factsheet.pdf.
- ↑ "Clopidogrel and interaction with proton pump inhibitors: comparison between cohort and within person study designs". BMJ 345. July 2012. doi:10.1136/bmj.e4388. PMID 22782731.
- ↑ "Concomitant use of clopidogrel and proton pump inhibitors: impact on platelet function and clinical outcome- a systematic review". Heart 99 (8): 520–527. April 2013. doi:10.1136/heartjnl-2012-302371. PMID 22851683.
- ↑ "Inhibition of CYP2C19 and CYP3A4 by omeprazole metabolites and their contribution to drug-drug interactions". Drug Metabolism and Disposition 41 (7): 1414–1424. July 2013. doi:10.1124/dmd.113.051722. PMID 23620487.
- ↑ "The drug-drug interaction between proton pump inhibitors and clopidogrel". CMAJ 180 (7): 699–700. March 2009. doi:10.1503/cmaj.090251. PMID 19332744.
- ↑ "Drug-drug interaction between clopidogrel and the proton pump inhibitors". The Annals of Pharmacotherapy 43 (7): 1266–1274. July 2009. doi:10.1345/aph.1M051. PMID 19470853.
- ↑ Selective Serotonin Reuptake Inhibitors and CYP2D6 at eMedicine
- ↑ "Pharmacogenetics of oral anticoagulants". Pharmacogenetics 13 (5): 247–252. May 2003. doi:10.1097/00008571-200305000-00002. PMID 12724615.
- ↑ 41.0 41.1 "Review article: comparison of the pharmacokinetics, acid suppression and efficacy of proton pump inhibitors". Alimentary Pharmacology & Therapeutics 14 (8): 963–978. August 2000. doi:10.1046/j.1365-2036.2000.00788.x. PMID 10930890.
- ↑ "Interaction of omeprazole, lansoprazole and pantoprazole with P-glycoprotein". Naunyn-Schmiedeberg's Archives of Pharmacology 364 (6): 551–557. December 2001. doi:10.1007/s00210-001-0489-7. PMID 11770010.
- ↑ "Interactions between herbal medicines and prescribed drugs: an updated systematic review". Drugs 69 (13): 1777–1798. 2009. doi:10.2165/11317010-000000000-00000. PMID 19719333.
- ↑ 44.0 44.1 "Clinical pharmacology of omeprazole". Clinical Pharmacokinetics 20 (1): 38–49. January 1991. doi:10.2165/00003088-199120010-00003. PMID 2029801.
- ↑ "Omeprazole: pharmacokinetics and metabolism in man". Scandinavian Journal of Gastroenterology. Supplement 166 (sup166): 33–40. January 1989. doi:10.3109/00365528909091241. PMID 2690330.
- ↑ 46.0 46.1 "Omeprazole. A preliminary review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in peptic ulcer disease and Zollinger-Ellison syndrome". Drugs 32 (1): 15–47. July 1986. doi:10.2165/00003495-198632010-00002. PMID 3527658.
- ↑ 47.0 47.1 47.2 47.3 "Omeprazole". https://www.drugbank.ca/drugs/DB00338.
- ↑ 48.0 48.1 "Prodrugs--from serendipity to rational design". Pharmacological Reviews 63 (3): 750–771. September 2011. doi:10.1124/pr.110.003459. PMID 21737530.
- ↑ "The chemically elegant proton pump inhibitors". American Journal of Pharmaceutical Education 70 (5): 101. October 2006. doi:10.5688/aj7005101. PMID 17149430.
- ↑ "Hydroxylation of R(+)- and S(-)-omeprazole after racemic dosing are different among the CYP2C19 genotypes". Pharmaceutical Research 29 (8): 2310–2316. August 2012. doi:10.1007/s11095-012-0757-x. PMID 22549736.
- ↑ "Influence of CYP2C19 pharmacogenetic polymorphism on proton pump inhibitor-based therapies". Drug Metabolism and Pharmacokinetics 20 (3): 153–167. June 2005. doi:10.2133/dmpk.20.153. PMID 15988117.
- ↑ "Nexium- esomeprazole magnesium capsule, delayed release; Nexium- esomeprazole magnesium granule, delayed release". 18 July 2023. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f4853677-1622-4037-688b-fdf533a11d96.
- ↑ "Omeprazole package insert". India: Dr. Reddy's Laboratories Limited. June 2013. https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=bced816b-6927-49fb-851f-f83a678dac97#nlm34090-1.
- ↑ "Guidelines for the diagnosis and management of gastroesophageal reflux disease". The American Journal of Gastroenterology 108 (3): 308–28; quiz 329. March 2013. doi:10.1038/ajg.2012.444. PMID 23419381.
- ↑ Baselt RC, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 1146–7. ISBN 978-0-9626523-7-0.
- ↑ "Trends in Drug Patenting - Case Studies: THE CASES: 5. OMEPRAZOLE". https://apps.who.int/medicinedocs/en/d/Js4915e/2.5.html.
- ↑ "Substituted benzimidazoles inhibit gastric acid secretion by blocking (H+ + K+)ATPase". Nature 290 (5802): 159–161. March 1981. doi:10.1038/290159a0. PMID 6259537. Bibcode: 1981Natur.290..159F.
- ↑ 58.0 58.1 "Making it easier to read prescriptions". FDA Consumer 29 (6): 25–27. July–August 1995. PMID 10143448. http://www.thebody.com/content/art13913.html. Retrieved 26 January 2011.
- ↑ "AstraZeneca/P&G's Prilosec OTC cleared by FDA for frequent heartburn" (in en). https://www.thepharmaletter.com/astrazeneca-p-g-s-prilosec-otc-cleared-by-fda-for-frequent-heartburn.
- ↑ Research, Center for Drug Evaluation and (2018-11-03). "Questions and Answers on Prilosec OTC (omeprazole)" (in en). FDA. https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/questions-and-answers-prilosec-otc-omeprazole.
- ↑ Research, Center for Drug Evaluation and (2018-11-03). "Prilosec OTC (omeprazole) Information" (in en). FDA. https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/prilosec-otc-omeprazole-information.
- ↑ Hummingbird Productions (2013-07-19). Prilosec OTC w/ Larry the Cable Guy. Retrieved 2025-07-25 – via YouTube.
- ↑ "Prilosec's Maker Switches Users to Nexium, Thwarting Generics". The Wall Street Journal. 6 June 2002. https://www.wsj.com/articles/SB1023326369679910840.
- ↑ "AstraZeneca awarded damages in Prilosec patent litigation". AstraZeneca (Press release). 3 December 2013. Retrieved 4 October 2023.
- ↑ "IN RE OMEPRAZOLE PATENT LITIGATION, Court of Appeals, Federal Circuit 2011". Google Scholar. https://scholar.google.com/scholar_case?case=12553883911671736992&q=+In+re+Omeprazole+Patent+Litigation&hl=en&as_sdt=40000003.
- ↑ "AstraZeneca to pay $425 mln to settle Nexium, Prilosec litigation in US". Reuters. 3 October 2023. https://www.reuters.com/legal/astrazeneca-pay-425-mln-settle-nexium-prilosec-litigation-us-2023-10-03/.
- ↑ "Omeprazole international". 3 February 2020. https://www.drugs.com/international/omeprazole.html.
- ↑ 68.0 68.1 "Omeprazole TriviumVet EPAR". 12 February 2025. https://www.ema.europa.eu/en/medicines/veterinary/EPAR/omeprazole-triviumvet#overview.
- ↑ "Omeprazole TriviumVet PI". 3 April 2025. https://ec.europa.eu/health/documents/community-register/html/v336.htm.
Further reading
- "Omeprazole Therapy and CYP2C19 Genotype". Medical Genetics Summaries. National Center for Biotechnology Information (NCBI). 2012. Bookshelf ID: NBK100895. https://www.ncbi.nlm.nih.gov/books/NBK100895/.
 |