Biology:Aldo-keto reductase
The aldo-keto reductase family is a family of proteins that are subdivided into 16 categories; these include a number of related monomeric NADPH-dependent oxidoreductases, such as aldehyde reductase, aldose reductase, prostaglandin F synthase, xylose reductase, rho crystallin, and many others.[1]
Structure
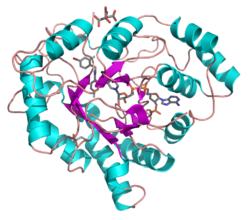
All possess a similar structure, with a beta-alpha-beta fold characteristic of nucleotide binding proteins.[2] The fold comprises a parallel beta-8/alpha-8-barrel, which contains a novel NADP-binding motif. The binding site is located in a large, deep, elliptical pocket in the C-terminal end of the beta sheet, the substrate being bound in an extended conformation. The hydrophobic nature of the pocket favours aromatic and apolar substrates over highly polar ones.[3]
Binding of the NADPH coenzyme causes a massive conformational change, reorienting a loop, effectively locking the coenzyme in place. This binding is more similar to FAD- than to NAD(P)-binding oxidoreductases.[4]
Examples
Some proteins of this family contain a potassium channel beta chain regulatory domain; these are reported to have oxidoreductase activity.[5]
See also
References
- ↑ "The aldo-keto reductase superfamily. cDNAs and deduced amino acid sequences of human aldehyde and aldose reductases". J. Biol. Chem. 264 (16): 9547–51. June 1989. doi:10.1016/S0021-9258(18)60566-6. PMID 2498333.
- ↑ "Sequence analysis of bovine lens aldose reductase". J. Biol. Chem. 265 (7): 3628–35. March 1990. doi:10.1016/S0021-9258(19)39639-5. PMID 2105951.
- ↑ "An unlikely sugar substrate site in the 1.65 A structure of the human aldose reductase holoenzyme implicated in diabetic complications". Science 257 (5066): 81–4. July 1992. doi:10.1126/science.1621098. PMID 1621098.
- ↑ "The crystal structure of the aldose reductase.NADPH binary complex". J. Biol. Chem. 267 (34): 24841–7. December 1992. doi:10.2210/pdb1abn/pdb. PMID 1447221.
- ↑ "Structure of the cytoplasmic beta subunit-T1 assembly of voltage-dependent K+ channels". Science 289 (5476): 123–7. July 2000. doi:10.1126/science.289.5476.123. PMID 10884227. Bibcode: 2000Sci...289..123G.
 |