Biology:ERCC2
TFIIH subunit XPD is a protein that in humans is encoded by the ERCC2 (ERCC excision repair 2) gene. It is a component of the general transcription and DNA repair factor IIH (TFIIH) core complex involved in transcription-coupled nucleotide excision repair.
Along with XPB, XPD is a part of human transcriptional initiation factor TFIIH and has ATP-dependent helicase activity.[1] It belongs to the RAD3/XPD subfamily of helicases.
The XPD (ERCC2) gene encodes for a 2.3-kb mRNA containing 22 exons and 21 introns. The XPD protein contains 760 amino acids and is a polypeptide with a size of 87kDa. Defects in this gene can result in three different disorders: the cancer-prone syndrome xeroderma pigmentosum complementation group D, photosensitive trichothiodystrophy, and Cockayne syndrome.[2]
XPD is essential for the viability of cells. Deletion of XPD in mice is lethal for developing embryos.[3]
XPD helicase is also employed in p53-mediated apoptotic cell death.[4]
Function
The ERCC2/XPD protein participates in nucleotide excision repair and is used in unwinding the DNA double helix after damage is initially identified. Nucleotide excision repair is a multi-step pathway that removes a wide range of different damages that distort normal base pairing. Such damages include bulky chemical adducts, ultraviolet-induced pyrimidine dimers, and several forms of oxidative damage.
The protein named XPD is expressed under the directions of the ERCC2 gene. The XPD protein is an indispensable part of the general transcription factor IIH (TFIIH) complex, which is a group of proteins. The two vital functions of the TFIIH complex are gene transcription and repairing damaged DNA. With the help of gene transcription, the TFIIH complex is able to control the functioning of many different genes in the body and the XPD protein acts as a stabilizer. XPB is another protein in the general transcription factor IIH (TFIIH) complex and is made from the ERCC3 gene, which works in coordination with XDP protein to commence the process of gene transcription.
Ultraviolet rays emerging from the sun, various hazardous chemicals, harmful radiations, are all known parameters for the sabotage of the DNA. A normal and healthy cell has the capability to fix the DNA damages before the problems begin due to the damaged DNA. Cells use nucleotide excision repair to fix damaged DNA. As a part of the process, the double-stranded DNA that encircles the damage is separated by the TFIIH complex. The XPD protein acts as a helicase and helps with the nucleotide excision repair process by binding to the specific regions of DNA and by unwinding the two DNA spiral strands. This exposes the damaged protein which allows the other proteins to remove the damaged section and replace the impaired area with the correct DNA.[5]
Clinical significance
Mutations
Mutations in the ERCC2/XPD gene can lead to various syndromes, either xeroderma pigmentosum (XP), trichothiodystrophy (TTD) or a combination of XP and TTD (XPTTD), or a combination of XP and Cockayne syndrome (XPCS).[6] TTD and CS both display features of premature aging. These features may include sensorineural deafness, retinal degeneration, white matter hypomethylation, central nervous system calcification, reduced stature, and cachexia (loss of subcutaneous fat tissue).[6][7] XPCS and TTD fibroblasts from ERCC2/XPD mutant human and mouse show evidence of defective repair of oxidative DNA damages that may underlie the segmental progeroid (premature aging) symptoms[8] (see DNA damage theory of aging).
Xeroderma pigmentosum
Xeroderma pigmentosum (XP) is associated with the lack of DNA repair mechanism and high susceptibility of cancer. A slight insufficiency in the DNA repair mechanism may result in the development of cancer. Some cancers have been recognized with the help of the relation between the single nucleotide polymorphism and genes. The XPD protein produced by the ERCC2 gene plays an important role in the process of transcription and cell death and is also known for nucleotide excision repair pathway. Various literature studies have reviewed the correlation between polymorphisms in ERCC2 and reduced DNA repair efficiency and their influence on the development of the cancers as well as interaction with environmental exposures.
The second most common cause of xeroderma pigmentosum in the United States are due to mutations in ERCC2 gene, more than twenty-five of which have been observed in people with this disease. The xeroderma pigmentosum is caused when the ERCC2 gene prevents the TFIIH complex from repairing the damaged DNA constructively.
Consequently, all the deformity collects inside the DNA, sabotaging the repair mechanism and results in the cancerous or dead cells. Thus, the people suffering from xeroderma pigmentosum are highly sensitive to the ultraviolet rays from the sunlight due to the DNA repair problems.
So, when ultraviolet rays harm the genes, the cell grows and divides in an uncontrolled fashion and is highly prone to be cancerous. Xeroderma pigmentosum have high risk of developing cancer in skin and eyes as they are the areas mostly exposed to sun. Xeroderma pigmentosum caused by ERCC2 mutations is associated with the numerable developmental neurological malfunctioning which includes; hearing loss, poor coordination, mobility issues, lack of intellectual abilities, difficulties in talking, walking, swallowing the food and seizures.
Researchers suspect that these neurological abnormalities are due to the accumulation of DNA damage despite the brain not being exposed to ultraviolet rays. Other factors might cause the DNA damage in nerve cells as well.[9]
Interactions
ERCC2 has been shown to interact with:
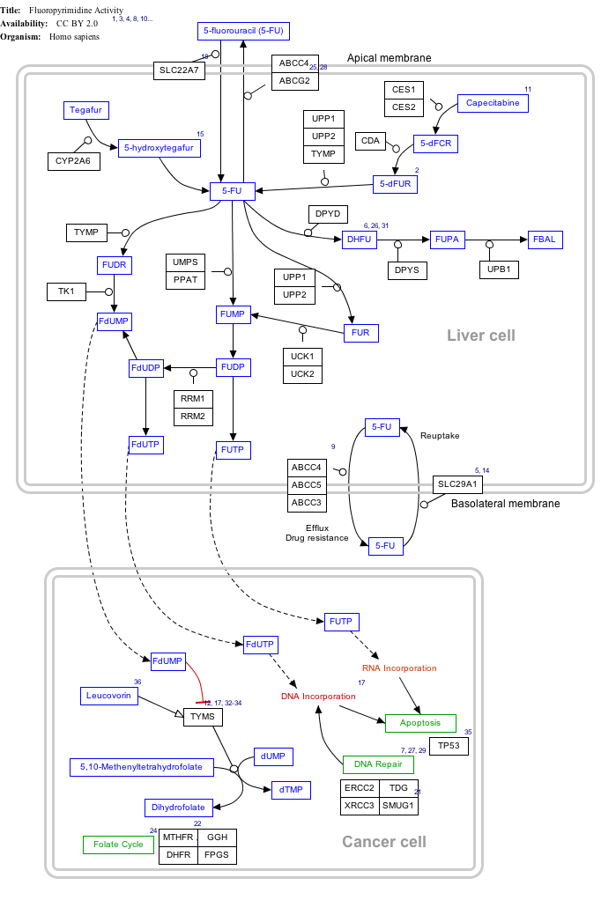
Interactive pathway map
Click on genes, proteins and metabolites below to link to respective articles.[§ 1]
- ↑ The interactive pathway map can be edited at WikiPathways: "FluoropyrimidineActivity_WP1601". http://www.wikipathways.org/index.php/Pathway:WP1601.
See also
References
- ↑ "Transcription of eukaryotic protein-coding genes". Annual Review of Genetics 34: 77–137. 2000. doi:10.1146/annurev.genet.34.1.77. PMID 11092823.
- ↑ "Entrez Gene: ERCC2 excision repair cross-complementing rodent repair deficiency, complementation group 2 (xeroderma pigmentosum D)". https://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=ShowDetailView&TermToSearch=2068.
- ↑ Liu, Jing. "XPD localizes in mitochondria and protects the mitochondrial genome from oxidative DNA damage". Nucleic Acids Research 43 (11).
- ↑ "p53-mediated apoptosis and genomic instability diseases". Acta Oncologica (Stockholm, Sweden) 40 (6): 696–701. 2001. doi:10.1080/02841860152619106. PMID 11765063.
- ↑ Reference, Genetics Home. "ERCC2 gene" (in en). https://medlineplus.gov/genetics/gene/ercc2/.
- ↑ 6.0 6.1 "Nucleotide excision repair disorders and the balance between cancer and aging". Cell Cycle 5 (24): 2886–8. 2006. doi:10.4161/cc.5.24.3565. PMID 17172862.
- ↑ "XPB and XPD helicases in TFIIH orchestrate DNA duplex opening and damage verification to coordinate repair with transcription and cell cycle via CAK kinase". DNA Repair (Amst.) 10 (7): 697–713. 2011. doi:10.1016/j.dnarep.2011.04.028. PMID 21571596.
- ↑ "An Xpd mouse model for the combined xeroderma pigmentosum/Cockayne syndrome exhibiting both cancer predisposition and segmental progeria". Cancer Cell 10 (2): 121–32. 2006. doi:10.1016/j.ccr.2006.05.027. PMID 16904611.
- ↑ Benhamou, Simone; Sarasin, Alain (2002-11-01). "ERCC2/XPD gene polymorphisms and cancer risk" (in en). Mutagenesis 17 (6): 463–469. doi:10.1093/mutage/17.6.463. ISSN 0267-8357. PMID 12435843. https://academic.oup.com/mutage/article/17/6/463/1274828.
- ↑ 10.0 10.1 "Interactions involving the human RNA polymerase II transcription/nucleotide excision repair complex TFIIH, the nucleotide excision repair protein XPG, and Cockayne syndrome group B (CSB) protein". Biochemistry 35 (7): 2157–67. Feb 1996. doi:10.1021/bi9524124. PMID 8652557.
- ↑ 11.0 11.1 "Dual role of TFIIH in DNA excision repair and in transcription by RNA polymerase II". Nature 368 (6473): 769–72. Apr 1994. doi:10.1038/368769a0. PMID 8152490. Bibcode: 1994Natur.368..769D.
- ↑ "Substrate specificity of the cdk-activating kinase (CAK) is altered upon association with TFIIH". The EMBO Journal 16 (7): 1628–37. Apr 1997. doi:10.1093/emboj/16.7.1628. PMID 9130708.
- ↑ "Mutations in the XPD helicase gene result in XP and TTD phenotypes, preventing interaction between XPD and the p44 subunit of TFIIH". Nature Genetics 20 (2): 184–8. Oct 1998. doi:10.1038/2491. PMID 9771713.
- ↑ "Sublimiting concentration of TFIIH transcription/DNA repair factor causes TTD-A trichothiodystrophy disorder". Nature Genetics 26 (3): 307–13. Nov 2000. doi:10.1038/81603. PMID 11062469.
- ↑ "A new, tenth subunit of TFIIH is responsible for the DNA repair syndrome trichothiodystrophy group A". Nature Genetics 36 (7): 714–9. Jul 2004. doi:10.1038/ng1387. PMID 15220921.
- ↑ "Cloning and characterization of p52, the fifth subunit of the core of the transcription/DNA repair factor TFIIH". The EMBO Journal 16 (5): 1093–102. Mar 1997. doi:10.1093/emboj/16.5.1093. PMID 9118947.
Further reading
- "Molecular and cellular analysis of the DNA repair defect in a patient in xeroderma pigmentosum complementation group D who has the clinical features of xeroderma pigmentosum and Cockayne syndrome". American Journal of Human Genetics 56 (1): 167–74. Jan 1995. PMID 7825573.
- "Tat, Tat-associated kinase, and transcription". Journal of Biomedical Science 5 (1): 24–7. 1998. doi:10.1007/BF02253352. PMID 9570510.
- "Transcriptional control: Tat cofactors and transcriptional elongation". Current Biology 8 (13): R447-9. Jun 1998. doi:10.1016/S0960-9822(98)70289-1. PMID 9651670. Bibcode: 1998CBio....8.R447Y.
- "A summary of mutations in the UV-sensitive disorders: xeroderma pigmentosum, Cockayne syndrome, and trichothiodystrophy". Human Mutation 14 (1): 9–22. 1999. doi:10.1002/(SICI)1098-1004(1999)14:1<9::AID-HUMU2>3.0.CO;2-6. PMID 10447254.
- "The xeroderma pigmentosum group D (XPD) gene: one gene, two functions, three diseases". Genes & Development 15 (1): 15–23. Jan 2001. doi:10.1101/gad.859501. PMID 11156600.
- "ERCC2/XPD gene polymorphisms and cancer risk". Mutagenesis 17 (6): 463–9. Nov 2002. doi:10.1093/mutage/17.6.463. PMID 12435843.
- "Polymorphisms in the human XPD (ERCC2) gene, DNA repair capacity and cancer susceptibility: an appraisal". DNA Repair 4 (10): 1068–74. Sep 2005. doi:10.1016/j.dnarep.2005.07.001. PMID 16054878.
External links
- GeneReviews/NIH/NCBI/UW entry on Xeroderma Pigmentosum
- ERCC2+Protein at the US National Library of Medicine Medical Subject Headings (MeSH)
 |