Biology:Nitrososphaerota
| Nitrososphaerota | |
|---|---|
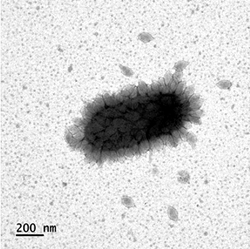
| |
| Nitrosopumilus maritimus, partially with virions of Nitrosopumilus spindle-shaped virus 1 (Thaspiviridae) attached. | |
| Scientific classification | |
| Domain: | |
| Superphylum: | |
| Phylum: | Nitrososphaerota Brochier-Armanet et al. 2021[1]
|
| Class: | Nitrososphaeria
|
| Order | |
| |
| Synonyms | |
| |
The Nitrososphaerota (syn. Thaumarchaeota) are a phylum of the Archaea proposed in 2008 after the genome of Cenarchaeum symbiosum was sequenced and found to differ significantly from other members of the hyperthermophilic phylum Thermoproteota (formerly Crenarchaeota).[3][2][4] Three described species in addition to C. symbiosum are Nitrosopumilus maritimus, Nitrososphaera viennensis, and Nitrososphaera gargensis.[2] The phylum was proposed in 2008 based on phylogenetic data, such as the sequences of these organisms' ribosomal RNA genes, and the presence of a form of type I topoisomerase that was previously thought to be unique to the eukaryotes.[2][5] This assignment was confirmed by further analysis published in 2010 that examined the genomes of the ammonia-oxidizing archaea Nitrosopumilus maritimus and Nitrososphaera gargensis, concluding that these species form a distinct lineage that includes Cenarchaeum symbiosum.[6] The lipid crenarchaeol has been found only in Nitrososphaerota, making it a potential biomarker for the phylum.[7][8] Most organisms of this lineage thus far identified are chemolithoautotrophic ammonia-oxidizers and may play important roles in biogeochemical cycles, such as the nitrogen cycle and the carbon cycle. Metagenomic sequencing indicates that they constitute ~1% of the sea surface metagenome across many sites.[9]
Nitrososphaerota-derived membrane-spanning tetraether lipids (glycerol dialkyl glycerol tetraethers; GDGTs) from marine sediments can be used to reconstruct past temperatures via the TEX86 paleotemperature proxy, as these lipids vary in structure according to temperature.[10] Because most Nitrososphaerota seem to be autotrophs that fix CO2, their GDGTs can act as a record for past Carbon-13 ratios in the dissolved inorganic carbon pool, and thus have the potential to be used for reconstructions of the carbon cycle in the past.[7]
Taxonomy
| Phylogeny of Nitrososphaerota[11][12][13] | |||||||||||||||||||||||||||||||||
|
| Phylogeny of Nitrososphaerota[14][15][16] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
The currently accepted taxonomy is based on the List of Prokaryotic names with Standing in Nomenclature (LPSN)[17] and National Center for Biotechnology Information (NCBI)[18]
- Class Nitrososphaeria Stieglmeier et al. 2014[19] [Conexivisphaeria Kato et al. 2020]
- ?"Cenoporarchaeum" corrig. Zhang et al. 2019
- ?"Candidatus Giganthauma" Muller et al. 2010[20]
- ?"Candidatus Nitrosodeserticola" Hwang et al. 2021
- Order "Geothermarchaeales" Adam et al. 2022
- Family Geothermarchaeaceae Adam et al. 2022
- ?"Geothermarchaeum" Adam et al. 2022
- ?"Scotarchaeum" Adam et al. 2022
- Family Geothermarchaeaceae Adam et al. 2022
- Order Conexivisphaerales Kato et al. 2020
- Family Conexivisphaeraceae Kato et al. 2020
- Conexivisphaera Kato et al. 2020
- Family Conexivisphaeraceae Kato et al. 2020
- Order "Nitrosocaldales" de la Torre et al. 2008
- Family "Nitrosocaldaceae" Qin et al. 2016
- "Candidatus Nitrosothermus" Luo et al. 2021
- "Candidatus Nitrosocaldus" de la Torre et al. 2008
- Family "Nitrosocaldaceae" Qin et al. 2016
- Order Nitrososphaerales Stieglmeier et al. 2014
- Family Methylarchaeaceae Hua et al. 2019
- ?"Candidatus Methylarchaeum" Hua et al. 2019
- ?"Candidatus Methanotowutia" Ou et al. 2022
- Family Nitrososphaeraceae Stieglmeier et al. 2014
- "Candidatus Nitrosocosmicus" Lehtovirta-Morley et al. 2016
- Nitrososphaera Stieglmeier et al. 2014[21]
- Family Methylarchaeaceae Hua et al. 2019
- Order Nitrosopumilales Qin et al. 2017[22]
- Family Nitrosopumilaceae Qin et al. 2017
- ?"Candidatus Nitrosospongia" Moeller et al. 2019
- "Candidatus Nitrosotalea" Lehtovirta 2011[23]
- "Candidatus Nitrosotenuis" Li et al. 2016[24][25]
- "Candidatus Nitrosopelagicus" Santoro et al. 2015[26]
- "Cenarchaeum" DeLong & Preston 1996
- Nitrosarchaeum corrig. Jung et al. 2018[27][28]
- Nitrosopumilus Qin et al. 2017[29][30][31]
- Family Nitrosopumilaceae Qin et al. 2017
Metabolism
Nitrososphaerota are important ammonia oxidizers in aquatic and terrestrial environments, and are the first archaea identified as being involved in nitrification.[32] They are capable of oxidizing ammonia at much lower substrate concentrations than ammonia-oxidizing bacteria, and so probably dominate in oligotrophic conditions.[8][33] Their ammonia oxidation pathway requires less oxygen than that of ammonia-oxidizing bacteria, so they do better in environments with low oxygen concentrations like sediments and hot springs. Ammonia-oxidizing Nitrososphaerota can be identified metagenomically by the presence of archaeal ammonia monooxygenase (amoA) genes, which indicate that they are overall more dominant than ammonia oxidizing bacteria.[8] In addition to ammonia, at least one Nitrososphaerota strain has been shown to be able to use urea as a substrate for nitrification. This would allow for competition with phytoplankton that also grow on urea.[34] One study of microbes from wastewater treatment plants found that not all Nitrososphaerota that express amoA genes are active ammonia oxidizers. These Nitrososphaerota may be capable of oxidizing methane instead of ammonia, or they may be heterotrophic, indicating a potential for a diversity of metabolic lifestyles within the phylum.[35] Marine Nitrososphaerota have also been shown to produce nitrous oxide, which as a greenhouse gas has implications for climate change. Isotopic analysis indicates that most nitrous oxide flux to the atmosphere from the ocean, which provides around 30% of the natural flux, may be due to the metabolic activities of archaea.[36]
Many members of the phylum assimilate carbon by fixing HCO3−.[9] This is done using a hydroxypropionate/hydroxybutyrate cycle similar to the Thermoproteota but which appears to have evolved independently. All Nitrososphaerota that have been identified by metagenomics thus far encode this pathway. Notably, the Nitrososphaerota CO2-fixation pathway is more efficient than any known aerobic autotrophic pathway. This efficiency helps explain their ability to thrive in low-nutrient environments.[33] Some Nitrososphaerota such as Nitrosopumilus maritimus are able to incorporate organic carbon as well as inorganic, indicating a capacity for mixotrophy.[9] At least two isolated strains have been identified as obligate mixotrophs, meaning they require a source of organic carbon in order to grow.[34]
A study has revealed that Nitrososphaerota are most likely the dominant producers of the critical vitamin B12. This finding has important implications for eukaryotic phytoplankton, many of which are auxotrophic and must acquire vitamin B12 from the environment; thus the Nitrososphaerota could play a role in algal blooms and by extension global levels of atmospheric carbon dioxide. Because of the importance of vitamin B12 in biological processes such as the citric acid cycle and DNA synthesis, production of it by the Nitrososphaerota may be important for a large number of aquatic organisms.[37]
Environment
Many Nitrososphaerota, such as Nitrosopumilus maritimus, are marine and live in the open ocean.[9] Most of these planktonic Nitrososphaerota, which compose the Marine Group I.1a, are distributed in the subphotic zone, between 100m and 350m.[7] Other marine Nitrososphaerota live in shallower waters. One study has identified two novel Nitrososphaerota species living in the sulfidic environment of a tropical mangrove swamp. Of these two species, Candidatus Giganthauma insulaporcus and Candidatus Giganthauma karukerense, the latter is associated with Gammaproteobacteria with which it may have a symbiotic relationship, though the nature of this relationship is unknown. The two species are very large, forming filaments larger than ever before observed in archaea. As with many Nitrososphaerota, they are mesophilic.[38] Genetic analysis and the observation that the most basal identified Nitrososphaerota genomes are from hot environments suggests that the ancestor of Nitrososphaerota was thermophilic, and mesophily evolved later.[32]
See also
References
- ↑ "Valid publication of the names of forty-two phyla of prokaryotes". Int J Syst Evol Microbiol 71 (10): 5056. 2021. doi:10.1099/ijsem.0.005056. PMID 34694987.
- ↑ 2.0 2.1 2.2 2.3 "Mesophilic Crenarchaeota: Proposal for a third archaeal phylum, the Thaumarchaeota". Nature Reviews Microbiology 6 (3): 245–52. March 2008. doi:10.1038/nrmicro1852. PMID 18274537.
- ↑ "Nitrososphaera viennensis, an ammonia oxidizing archaeon from soil". Proceedings of the National Academy of Sciences of the United States of America 108 (20): 8420–5. May 2011. doi:10.1073/pnas.1013488108. PMID 21525411. Bibcode: 2011PNAS..108.8420T.
- ↑ DeLong, E. F. (1992-06-15). "Archaea in coastal marine environments.". Proceedings of the National Academy of Sciences 89 (12): 5685–5689. doi:10.1073/pnas.89.12.5685. ISSN 0027-8424. PMID 1608980. Bibcode: 1992PNAS...89.5685D.
- ↑ "A DNA topoisomerase IB in Thaumarchaeota testifies for the presence of this enzyme in the last common ancestor of Archaea and Eucarya". Biology Direct 3: 54. December 2008. doi:10.1186/1745-6150-3-54. PMID 19105819.
- ↑ "Distinct gene set in two different lineages of ammonia-oxidizing archaea supports the phylum Thaumarchaeota". Trends in Microbiology 18 (8): 331–40. August 2010. doi:10.1016/j.tim.2010.06.003. PMID 20598889.
- ↑ 7.0 7.1 7.2 "Stable carbon isotope ratios of intact GDGTs indicate heterogeneous sources to marine sediments". Geochimica et Cosmochimica Acta 181: 18–35. 2016. doi:10.1016/j.gca.2016.02.034. Bibcode: 2016GeCoA.181...18P.
- ↑ 8.0 8.1 8.2 "The Thaumarchaeota: an emerging view of their phylogeny and ecophysiology". Current Opinion in Microbiology 14 (3): 300–6. June 2011. doi:10.1016/j.mib.2011.04.007. PMID 21546306.
- ↑ 9.0 9.1 9.2 9.3 "Nitrosopumilus maritimus genome reveals unique mechanisms for nitrification and autotrophy in globally distributed marine crenarchaea". Proceedings of the National Academy of Sciences of the United States of America 107 (19): 8818–23. May 2010. doi:10.1073/pnas.0913533107. PMID 20421470. Bibcode: 2010PNAS..107.8818W.
- ↑ "Distributional variations in marine crenarchaeotal membrane lipids: a new tool for reconstructing ancient sea water temperatures?". Earth and Planetary Science Letters 204 (1–2): 265–274. 2002. doi:10.1016/S0012-821X(02)00979-2. Bibcode: 2002E&PSL.204..265S.
- ↑ "The LTP". https://imedea.uib-csic.es/mmg/ltp/#LTP.
- ↑ "LTP_all tree in newick format". https://imedea.uib-csic.es/mmg/ltp/wp-content/uploads/ltp/LTP_all_06_2022.ntree.
- ↑ "LTP_06_2022 Release Notes". https://imedea.uib-csic.es/mmg/ltp/wp-content/uploads/ltp/LTP_06_2022_release_notes.pdf.
- ↑ "GTDB release 08-RS214". https://gtdb.ecogenomic.org/about#4%7C.
- ↑ "ar53_r214.sp_label". https://data.gtdb.ecogenomic.org/releases/release214/214.0/auxillary_files/ar53_r214.sp_labels.tree.
- ↑ "Taxon History". https://gtdb.ecogenomic.org/taxon_history/.
- ↑ J.P. Euzéby. "Thaumarchaeota". List of Prokaryotic names with Standing in Nomenclature (LPSN). https://lpsn.dsmz.de/phylum/thaumarchaeota. Retrieved 2021-03-20.
- ↑ Sayers. "Thaumarchaeota". National Center for Biotechnology Information (NCBI) taxonomy database. https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Tree&id=651137&lvl=3&keep=1&srchmode=1&unlock. Retrieved 2021-03-20.
- ↑ "Nitrososphaera viennensis gen. nov., sp. nov., an aerobic and mesophilic, ammonia-oxidizing archaeon from soil and a member of the archaeal phylum Thaumarchaeota". International Journal of Systematic and Evolutionary Microbiology 64 (Pt 8): 2738–52. August 2014. doi:10.1099/ijs.0.063172-0. PMID 24907263.
- ↑ "First description of giant Archaea (Thaumarchaeota) associated with putative bacterial ectosymbionts in a sulfidic marine habitat.". Environmental Microbiology 12 (8): 2371–83. August 2010. doi:10.1111/j.1462-2920.2010.02309.x. PMID 21966926. Bibcode: 2010EnvMi..12.2371M.
- ↑ "Genome sequence of Candidatus Nitrososphaera evergladensis from group I.1b enriched from Everglades soil reveals novel genomic features of the ammonia-oxidizing archaea". PLOS ONE 9 (7): e101648. 7 July 2014. doi:10.1371/journal.pone.0101648. PMID 24999826. Bibcode: 2014PLoSO...9j1648Z.
- ↑ "Isolation of an autotrophic ammonia-oxidizing marine archaeon". Nature 437 (7058): 543–6. September 2005. doi:10.1038/nature03911. PMID 16177789. Bibcode: 2005Natur.437..543K.
- ↑ "Cultivation of an obligate acidophilic ammonia oxidizer from a nitrifying acid soil". Proceedings of the National Academy of Sciences of the United States of America 108 (38): 15892–7. September 2011. doi:10.1073/pnas.1107196108. PMID 21896746. Bibcode: 2011PNAS..10815892L.
- ↑ "Enrichment and genome sequence of the group I.1a ammonia-oxidizing Archaeon "Ca. Nitrosotenuis uzonensis" representing a clade globally distributed in thermal habitats". PLOS ONE 8 (11): e80835. 2013. doi:10.1371/journal.pone.0080835. PMID 24278328. Bibcode: 2013PLoSO...880835L.
- ↑ "A novel ammonia-oxidizing archaeon from wastewater treatment plant: Its enrichment, physiological and genomic characteristics". Scientific Reports 6: 23747. March 2016. doi:10.1038/srep23747. PMID 27030530. Bibcode: 2016NatSR...623747L.
- ↑ "Genomic and proteomic characterization of "Candidatus Nitrosopelagicus brevis": an ammonia-oxidizing archaeon from the open ocean". Proceedings of the National Academy of Sciences of the United States of America 112 (4): 1173–8. January 2015. doi:10.1073/pnas.1416223112. PMID 25587132. Bibcode: 2015PNAS..112.1173S.
- ↑ "Genome of a low-salinity ammonia-oxidizing archaeon determined by single-cell and metagenomic analysis". PLOS ONE 6 (2): e16626. February 2011. doi:10.1371/journal.pone.0016626. PMID 21364937. Bibcode: 2011PLoSO...616626B.
- ↑ "Genome sequence of an ammonia-oxidizing soil archaeon, "Candidatus Nitrosoarchaeum koreensis" MY1". Journal of Bacteriology 193 (19): 5539–40. October 2011. doi:10.1128/JB.05717-11. PMID 21914867.
- ↑ "Draft genome sequence of an ammonia-oxidizing archaeon, "Candidatus Nitrosopumilus koreensis" AR1, from marine sediment". Journal of Bacteriology 194 (24): 6940–1. December 2012. doi:10.1128/JB.01857-12. PMID 23209206.
- ↑ "Genome sequence of "Candidatus Nitrosopumilus salaria" BD31, an ammonia-oxidizing archaeon from the San Francisco Bay estuary". Journal of Bacteriology 194 (8): 2121–2. April 2012. doi:10.1128/JB.00013-12. PMID 22461555.
- ↑ "Physiological and genomic characterization of two novel marine thaumarchaeal strains indicates niche differentiation". The ISME Journal 10 (5): 1051–63. May 2016. doi:10.1038/ismej.2015.200. PMID 26528837. Bibcode: 2016ISMEJ..10.1051B.
- ↑ 32.0 32.1 "Spotlight on the Thaumarchaeota". The ISME Journal 6 (2): 227–30. February 2012. doi:10.1038/ismej.2011.145. PMID 22071344. Bibcode: 2012ISMEJ...6..227B.
- ↑ 33.0 33.1 "Ammonia-oxidizing archaea use the most energy-efficient aerobic pathway for CO2 fixation". Proceedings of the National Academy of Sciences of the United States of America 111 (22): 8239–44. June 2014. doi:10.1073/pnas.1402028111. PMID 24843170. Bibcode: 2014PNAS..111.8239K.
- ↑ 34.0 34.1 Qin, Wei; Amin, Shady A.; Martens-Habbena, Willm; Walker, Christopher B.; Urakawa, Hidetoshi; Devol, Allan H.; Ingalls, Anitra E.; Moffett, James W. et al. (2014). "Marine ammonia-oxidizing archaeal isolates display obligate mixotrophy and wide ecotypic variation". Proceedings of the National Academy of Sciences 111 (34): 12504–12509. doi:10.1073/PNAS.1324115111. ISSN 0027-8424. PMID 25114236. Bibcode: 2014PNAS..11112504Q.
- ↑ "Thaumarchaeotes abundant in refinery nitrifying sludges express amoA but are not obligate autotrophic ammonia oxidizers". Proceedings of the National Academy of Sciences of the United States of America 108 (40): 16771–6. October 2011. doi:10.1073/pnas.1106427108. PMID 21930919. Bibcode: 2011PNAS..10816771M.
- ↑ Santoro, A. E.; Buchwald, C.; McIlvin, M. R.; Casciotti, K. L. (2011-09-02). "Isotopic Signature of N2O Produced by Marine Ammonia-Oxidizing Archaea". Science 333 (6047): 1282–1285. doi:10.1126/science.1208239. ISSN 0036-8075. PMID 21798895. Bibcode: 2011Sci...333.1282S.
- ↑ "Aquatic metagenomes implicate Thaumarchaeota in global cobalamin production". The ISME Journal 9 (2): 461–71. February 2015. doi:10.1038/ismej.2014.142. PMID 25126756. Bibcode: 2015ISMEJ...9..461D.
- ↑ "First description of giant Archaea (Thaumarchaeota) associated with putative bacterial ectosymbionts in a sulfidic marine habitat". Environmental Microbiology 12 (8): 2371–83. August 2010. doi:10.1111/j.1462-2920.2010.02309.x. PMID 21966926. Bibcode: 2010EnvMi..12.2371M.
Further reading
- "Microbial community stratification controlled by the subseafloor fluid flow and geothermal gradient at the Iheya North hydrothermal field in the Mid-Okinawa Trough (Integrated Ocean Drilling Program Expedition 331)". Applied and Environmental Microbiology 80 (19): 6126–35. October 2014. doi:10.1128/AEM.01741-14. PMID 25063666. Bibcode: 2014ApEnM..80.6126Y.
- "Ammonia oxidation-dependent growth of group I.1b Thaumarchaeota in acidic red soil microcosms". FEMS Microbiology Ecology 89 (1): 127–34. July 2014. doi:10.1111/1574-6941.12340. PMID 24724989. Bibcode: 2014FEMME..89..127W.
- "Pangenome evidence for extensive interdomain horizontal transfer affecting lineage core and shell genes in uncultured planktonic thaumarchaeota and euryarchaeota". Genome Biology and Evolution 6 (7): 1549–63. June 2014. doi:10.1093/gbe/evu127. PMID 24923324.
Wikidata ☰ Q1186957 entry
