Biology:Prephenate dehydrogenase
| prephenate dehydrogenase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
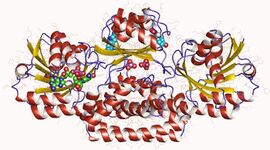 Prephenate dehydrogenase homodimer, Bacillus anthracis | |||||||||
| Identifiers | |||||||||
| EC number | 1.3.1.12 | ||||||||
| CAS number | 9044-92-2 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
Prephenate dehydrogenase is an enzyme found in the shikimate pathway, and helps catalyze the reaction from prephenate to tyrosine.
Nomenclature
Gene: (Saccharomyces Cerevisiae) TYR1[1]
Shikimate pathway: Arogenate/Prephenate (ADH/PDH). Although in the shikimate pathway arogenate and prephenate dehydrogenase catalyze different reactions, they can at times be used interchangeably.[2]
- TyrA (tyrosine A: within the tyrosine pathway)[3]
- Prephenate dehydrogenase[4]
- Prephenate (Nicotinamide adenine dinucleotide phosphate) dehydrogenase[5]
- Prephenate dehydrogenase (NADP)[6]
- NADP+ oxidoreductase[7]
This enzyme so far has been found in sixteen different organisms; twelve different kinds of bacteria (mostly cyanobacteria) and four different kinds of plants (mostly different kinds of beans).[8]
Bacteria organisms (examples): Acenitobacter calcoaceticus, Fischerella sp., Flavobacterium so., Comamonas testosteroni, and nostoc sp.
Plant organisms: phaseolus coccineus, phaseolus vulgaris, vicia faba, vigna radiata
Function
Present in the shikimate pathway, in the pathway to synthesize tyrosine (a non-essential amino acid in both plants and animals). It catalyzes the oxidative decarboxylation reaction of prephenate to 4-hydroxyphenylpyruvate.[9]
Reaction
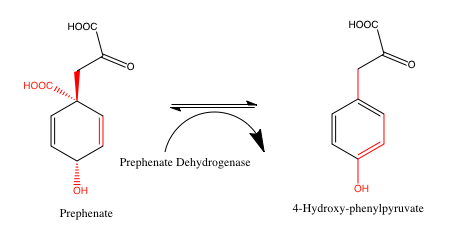
In enzymology, a prephenate dehydrogenase (EC 1.3.1.12) is an enzyme that catalyzes the chemical reaction
- prephenate + NAD+ 4-hydroxyphenylpyruvate + CO2 + NADH
Thus, the two substrates of this enzyme are prephenate and NAD+, whereas its 3 products are 4-hydroxyphenylpyruvate, CO2, and NADH.
Structure
This enzyme belongs to the family of oxidoreductases, specifically those acting on the CH-CH group of donor with NAD+ or NADP+ as acceptor. The systematic name of this enzyme class is prephenate:NAD+ oxidoreductase (decarboxylating). Other names in common use include hydroxyphenylpyruvate synthase, and chorismate mutase---prephenate dehydrogenase. This enzyme participates in phenylalanine, tyrosine and tryptophan biosynthesis and novobiocin biosynthesis.
Also found in haemophilus influenzae, synechocystis (bacteria), and aquifex aeolicus (plant).
However, in haemophilus influenzae, prephenate dehydrogenase is fused with the enzyme chorismate mutase. This fusion is not found in plants or animals.[10]
Structural studies
As of late 2007, two structures have been solved for this class of enzymes, with PDB accession codes 2G5C and 2PV7.
References
- ↑ "Characterization of the Prephenate Dehydrogenase-encoding Gene, TYR1, from Saccharomyces Cerevisiae". Gene 85 (2): 303–11. 1989. doi:10.1016/0378-1119(89)90422-8. PMID 2697638.
- ↑ "EC 1.3.1.13." EC 1.3.1.13. IUBMB Enzyme Nomenclature, 1972. Web. 24 Apr. 2014.
- ↑ "Prephenate Dehydrogenase - TyrA - Bacillus Subtilis (strain 168)."Prephenate Dehydrogenase - TyrA - Bacillus Subtilis (strain 168). UniProt, 13 Nov. 2013. Web. 24 Apr. 2014.
- ↑ "EC 1.3.1.13." EC 1.3.1.13. IUBMB Enzyme Nomenclature, 1972. Web. 24 Apr. 2014.
- ↑ "EC 1.3.1.13." EC 1.3.1.13. IUBMB Enzyme Nomenclature, 1972. Web. 24 Apr. 2014.
- ↑ "EC 1.3.1.13." EC 1.3.1.13. IUBMB Enzyme Nomenclature, 1972. Web. 24 Apr. 2014.
- ↑ "EC 1.3.1.13." EC 1.3.1.13. IUBMB Enzyme Nomenclature, 1972. Web. 24 Apr. 2014.
- ↑ "EC 1.3.1.13 - Prephenate Dehydrogenase (NADP+)." Information on Prephenate Dehydrogenase. BRENDA, n.d. Web. 24 Apr. 2014.
- ↑ "InterPro." Bifunctional Chorismate Mutase/prephenate Dehydrogenase T-protein (IPR008244). InterPro, n.d. Web. 24 Apr. 2014.
- ↑ "The Structure of Haemophilus Influenzae Prephenate Dehydrogenase Suggests Unique Features of Bifunctional TyrA Enzymes". Acta Crystallogr F 66 (Pt 10): 1317–25. Oct 2010. doi:10.1107/S1744309110021688. PMID 20944228.
 |

