Biology:Symbiotic theory of cellular evolution
Symbiotic theory of cellular evolution is a theory that postulates symbiosis as an evolutionary mechanism other than Darwinism solely. Symbiosis refers to the interaction between two or more organisms in a mutualistic, commensal or parasitic manner. Organisms can be divided into two types, prokaryotes and eukaryotes, with the latter being more complex.
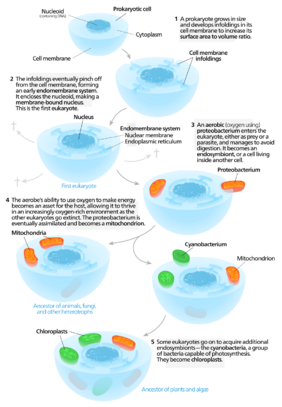
This theory was proposed by Russian biologist Constantin Mereschkowsky in 1905 and refined by American microbiologist Lynn Margulis in 1967. It is used to explain the biogenesis of double-membrane bounded organelles like plastids and mitochondria. It also serves as a basis for models of eukaryogenesis, the evolution of eukaryotes from prokaryotes. The timing of eukaryogenesis is dependent on microfossil and molecular dating, and the mechanism of eukaryogenesis is hypothesized by four models: Phagocytosing archaeon model, Hydrogen model, Syntrophy model and Late mitochondrial endosymbiosis model.
History
Russian biologist Constantin Mereschkowsky was the first to provide solid arguments that some cells come from an intracellular union of two different types of cells (endosymbiosis). These arguments about the origin of plastids included three essential ideas in Mereschkowsky's article published in 1905:[1]
- Plastids that resembled ancient cyanobacteria established a symbiosis with a heterotrophic host
- A large heterotrophic, amoeboid-type host cell combined with a smaller, micrococcus-type endosymbiont that formed the nucleus to form the host of the plastids.
- The autotrophy of plants is entirely inherited from cyanobacteria
However, the article has no inclusion of the origin of mitochondria. The endosymbiotic origin of mitochondria is first proposed by the French microbiologist Paul Portier.[2]
With microbiologists rejecting endosymbiotic theory throughout the 1920s to 60s,[3] the proposed theories received no more attention from scientists then after. The theory of endosymbiotic origin of organelles in the eukaryotic cell gained attention again in 1967 by the American microbiologist Lynn Margulis due to the development of new methods such as electron microscopy, molecular biology and biochemistry to study cells. Using more modern technology, she made the first suggestions that both plastids and mitochondria in the endosymbiotic theory evolved from separate endosymbionts.[4]
Symbiotic organelle biogenesis
Mitochondria
In aerobic eukaryotes, the mitochondria, which has the ability to convert biochemical energy from nutrients to ATP utilizing respiration, is one of few organelles possessing its own genome. The discovery of the presence of a small genome within mitochondria sparked research interest. Such researches showed RNA sequence similarities between mitochondrial and bacterial rRNAs,[5] and the differences between that of the nucleus prompted biologists to speculate that mitochondria were of endosymbiotic organismic origin. Subsequent analyses showed that all eukaryotic mitochondria originated from a single ancestor, Proteobacteria.[6]
The postulation of the symbiotic theory of cellular evolution is that eukaryotes emerged from a symbiotic association of prokaryotic cells. Fusion of prokaryotes and the integration of mitochondria potentiated the transition of cells from being prokaryotic to eukaryotic [7]
However, the nature of the original host cell remains a controversial issue. One of the possible hypotheses in deducing its nature is the hydrogen hypothesis which hypothesized the establishment of a stable symbiotic relationship between an archaea and an α-proteobacteria.[8] According to the hypothesis, the host is a hydrogen-dependent archaea while the symbiote is an anaerobic organism like α-proteobacteria which can either breathe in the presence of O2 or perform H2-producing fermentations under anaerobic conditions. The production of energy by the latter metabolic pathway has to be dependent on a low concentration of H2, which can be provided by the association of a H2-dependent archaea.[3] The strengths of this hypothesis include:
- The cross-feed nature of partners created mutual dependence between the archaeon host and the bacterial symbiote
- The archaea and bacteria involved are present in the current biosphere[3]
The mutualistic interaction between the bacteria and archaea rendered the retainment of the bacterial symbiote by the archaeon host. The retainment is first initiated by the transferral of pre-existing genes of the symbiont encoding importers and heterotrophic carbon metabolic proteins to the chromosomes of the nucleoid of the archaeon host for gene expression. Constant expression of endosymbiotic genes replaced indigenous archaeon metabolic pathways, transforming the archaeon from within. As a result, the archaeon is transformed from an autotroph and to a heterotroph.[3]
By this theory, during the Precambrian period, all currently known eukaryotes descended from an archaeon ancestor which acquired a proteobacterium that became the mitochondria. The integration of the mitochondria provides cells sufficient energy resources for the complex development and overcome the strong energy constraint in the prokaryotes.This process marks the emergence of the eukaryotic cell and evolution [9]
The establishment of this stage in these cells is a prerequisite for the acquisition of eukaryotic cellular characteristics and eventually integrated cyanobacteria.[3] Moreover, the acquisition of these bacterial-derived organelles served major roles in metabolism (e.g. steroid hormone synthesis, b-oxidation of fatty acids) and signaling (e.g. calcium signaling, apoptosis,) which enhanced cellular efficiency and capacity for complex interactions mediation.[9] Therefore, the energy constraint is also the reason behind the lack of real intermediaries in the transition from prokaryotes to eukaryotes.
Research on the age of mitochondrial symbiosis revealed many genes. Some of these genes which contributed to the sophisticated structure of eukaryotes were most likely obtained before mitochondria. The acquisition of complex eukaryotic characteristics prior to mitochondrial entrance points to the possibility that the development of eukaryotic complexity had commenced without the mitochondria. Due to such possibilities, the role of mitochondrial entrance in eukaryogenesis remains speculative.[9]
Plastids
Photosynthesis is the process by which plants or other organisms utilize sunlight for the oxidation of water and reduction of carbon dioxide to produce glucose and oxygen. This process was only available for organisms with the pigment called chlorophyll and was thus exclusive to one lineage of bacteria, cyanobacteria. The endocytosis of a cyanobacterium [1][4] gave other organisms the ability to conduct photosynthesis. This evolutionary event known as primary endocytosis, which is the single origin of all eukaryotic plastids, was discovered through phylogenetic analysis of plastid-encoded genes.[10][10] Phylogenetic researches analyzing the origin of the concerned cyanobacterial endosymbiont concluded it belonged to the Gloeomargaritales order.[6]
Primary endocytosis was hypothesized to occur due to predation of cyanobacteria by heterotrophs. These heterotrophs first capture cyanobacteria via a process known as phagocytosis, then digest the bacteria with its enzymes. However, an unknown mechanism of the cyanobacteria allowed evasion of digestion. The avoidance of destruction by digestive enzymes allowed the maintenance of a stable metabolic relationship. This interdependency between the host and the cyanobacterium facilitated internalization, where the inner membrane of the new organelle was contributed by the bacteria and the outer membrane by the endocytic vesicle of the host.[11] Following internalization, the mediation of a gene transfer from the bacterium to the nucleus of the host called endosymbiotic gene transfer[8] completes the process of primary endocytosis.
The primary endocytosis diversified the organisms able to conduct photosynthesis and gave rise to three groups, the Viridiplantae (green algae and land plants), Rhodophyta (red algae), and Glaucophyta (glaucophyte algae) which form the monophyletic supergroup called Archaeplastida.[12][13] However these three groups did not account for all photosynthetic organisms. The origin of these other lineages of photosynthetic organisms were found not to be from primary endocytosis but from secondary endocytosis, a process that is mediated through the endocytosis of a eukaryotic host with plastids by another eukaryotic cell. This marks the origin of an array of algal species including the euglenids and chlorarachniophytes containing green plastids, the haptophytes, stramenopiles, dinoflagellates, cryptophytes (diatoms, brown and golden algae, etc.) with red plastids.[6] It also gave rise to the Apicomplexa, a major group of parasites, in which the plastid has lost its photosynthetic function and become an apicoplast, an example being the Plasmodium, the cause of malaria.[14]
Eukaryogenesis
When did eukaryotes evolve
Two lineages of symbiotic organism exist: the crown lineage comprising all the lineages descended from the last eukaryotic common ancestor (LECA), and the stem lineage comprising all the diverging lineages before the evolution of the LECA, which are all extinct. The existence of these two lineages creates a problem for using geochemical and fossil records to determine when eukaryotes evolved because any characteristically eukaryotic feature of the microfossils is not indicative of whether the organism is of stem or crown origin.[6] The use of microfossils to determine when the LECA evolved depends on the existence of specific features that are particular to the biomarkers or fossils associated definitively with certain eukaryotic groups to differentiate crown from stem lineages. Such specific features of microfossils include large size, decorated walls and complex ultrastructure, all of which make an individual more likely to be eukaryotic that prokaryotic.[15]
Currently, the oldest microfossil of possible eukaryotic origin dates back to 3200 million years ago, yet the lack of a decorated ultrastructure prevents the time frame from being a definitive time of evolution.[16] The oldest fossil displaying evident structural features of eukaryotic affiliation dates back to around 1700 million years ago,[17] showing a major gap of the starting point of eukaryogenesis.
Apart from the usage of microfossils, molecular dating like “relaxed-molecular clock” methods are used as complementary approaches to determine the timing of eukaryotic evolution, yet the results remain varied,[6] from between 1866 and 1679 million years ago [18] to 950 and 1,259 million years ago.[19] However, this approach is dependent on the assumption that mitochondrial endosymbiosis is the triggering of eukaryogenesis and that eukaryogenesis occurred long before the evolution of the LECA. This approach is not useful when assuming mitochondrial endosymbiosis as an unimportant event in eukaryogenesis as the endosymbiosis occurs around the time of the evolution of the LECA.[6]
How did eukaryotes evolve
The symbiotic theory of cellular evolution serves as the basis for eukaryogenesis. Theorized mechanisms for eukaryogenesis include the phagocytosing archezoa model, the hydrogen model, the syntrophy model and the late mitochondrial endosymbiosis model.
Phagocytosing archezoa model
The phagocytosing archezoa model theorizes the phagocytosis of an α-proteobacterium by a eukaryotic-like host cell that has a nucleus but not a mitochondria, which the bacterium then transforms into the mitochondrion,[20] marks the start of eukaryogenesis. This model is supported by the existence of Archezoa, an anaerobic eukaryote with no mitochondria, and is composed of two independent hypotheses: a protoeukaryote host engulfed the mitochondrial ancestor, and that modern Archezoa are the 'missing links' that never possessed mitochondria. However, the second hypothesis has been disproved by the emergence of discoveries that many Archezoa contain mitochondrial symbiont-derived genes, meaning that their evolution occurs after mitochondrial symbiosis.[21]
Hydrogen model
The hydrogen model postulates that the first eukaryotic cell is generated by a fusion between an α-proteobacterium and an archaeal cell, with the α-proteobacterial subsequently evolving into the mitochondrion. (See the section Mitochondria) In this hydrogen model scenario, it is assumed that any bacterial genetic signal in the nuclear genome is predominantly α-proteobacterial. However, genetic analyses revealed that the bacterial-type genes in eukaryotes cannot be affiliated with any specific bacterial phylum and that they are derived from diverse non-α-proteobacterial lineages.[22]
Syntrophy model
The syntrophy model postulates that two independent endosymbiotic events involving a myxobacterium, a methanogenic archaeon, and an alpha proteobacterium produce the eukaryotes.
According to this model, the uptake of liberated hydrogen, carbon dioxide and acetate by a myxobacterium through the fermentation of organic substances mediated by an methanogen establishes a symbiotic relationship under anaerobic conditions. This symbiotic relationship further deepens through the development of substantial cell-cell contact for metabolite exchange. The increase in cell-cell contact led to the eventual encircling of the methanogen and fusion of membranes around it via phagocytosis-like mechanisms. The entrance of the methanogen caused its evolution into the nucleus due to its provision of most of the genetic machinery, while the myxobacteria, characterized by complex life cycles and cell-to-cell communication, was transformed into the cytoplasm. After the first endosymbiosis, the second symbiosis incorporating the α-proteobacterium, which evolved into the mitochondria, into the syntrophic consortium [23] marks the start of eukaryogenesis.
Late mitochondrial endosymbiosis model
This hypothesis uses phylogenomics to construct the chronology of the acquisition of proto-mitochondrial proteins. It postulates multiple serial endosymbiosis with the participation of multiple bacteria that experienced endosymbiotic gene transfer marks the start of eukaryogenesis, with the endosymbiosis of α-proteobacterium as the ultimate step.[6][24]
References
- ↑ 1.0 1.1 Martin, William; Kowallik, Klaus (August 1999). "Annotated English translation of Mereschkowsky's 1905 paper 'Über Natur und Ursprung der Chromatophoren imPflanzenreiche'" (in en). European Journal of Phycology 34 (3): 287–295. doi:10.1080/09670269910001736342. ISSN 0967-0262. http://www.tandfonline.com/doi/abs/10.1080/09670269910001736342.
- ↑ "Les Symbiotes". Nature 103 (2599): 482–483. August 1919. doi:10.1038/103482b0. ISSN 0028-0836. Bibcode: 1919Natur.103..482.. http://dx.doi.org/10.1038/103482b0.
- ↑ 3.0 3.1 3.2 3.3 3.4 Martin, William F.; Garg, Sriram; Zimorski, Verena (2015-09-26). "Endosymbiotic theories for eukaryote origin" (in en). Philosophical Transactions of the Royal Society B: Biological Sciences 370 (1678): 20140330. doi:10.1098/rstb.2014.0330. ISSN 0962-8436. PMID 26323761.
- ↑ 4.0 4.1 Sagan, Lynn (March 1967). "On the origin of mitosing cells". Journal of Theoretical Biology 14 (3): 225–IN6. doi:10.1016/0022-5193(67)90079-3. ISSN 0022-5193. PMID 11541392. Bibcode: 1967JThBi..14..225S. http://dx.doi.org/10.1016/0022-5193(67)90079-3.
- ↑ Bonen, L.; Cunningham, R.S.; Gray, M.W.; Doolittle, W.F. (1977). "Wheat embryo mitochondrial 18S ribosomal RNA: evidence for its prokaryotic nature" (in en). Nucleic Acids Research 4 (3): 663–671. doi:10.1093/nar/4.3.663. ISSN 0305-1048. PMID 866186.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 López-García, Purificación; Eme, Laura; Moreira, David (December 2017). "Symbiosis in eukaryotic evolution" (in en). Journal of Theoretical Biology 434: 20–33. doi:10.1016/j.jtbi.2017.02.031. PMID 28254477. Bibcode: 2017JThBi.434...20L.
- ↑ "Symbiosis and evolution: at the origin of the eukaryotic cell" (in en-US). 2016-06-23. https://www.encyclopedie-environnement.org/en/life/symbiosis-and-evolution-origin-eukaryotic-cell/.
- ↑ 8.0 8.1 Martin, William; Müller, Miklós (March 1998). "The hydrogen hypothesis for the first eukaryote" (in en). Nature 392 (6671): 37–41. doi:10.1038/32096. ISSN 0028-0836. PMID 9510246. Bibcode: 1998Natur.392...37M. http://www.nature.com/articles/32096.
- ↑ 9.0 9.1 9.2 Douglas, A. E. (2014-02-01). "Symbiosis as a General Principle in Eukaryotic Evolution" (in en). Cold Spring Harbor Perspectives in Biology 6 (2): a016113. doi:10.1101/cshperspect.a016113. ISSN 1943-0264. PMID 24492707.
- ↑ 10.0 10.1 Archibald, John M. (January 2009). "The Puzzle of Plastid Evolution" (in en). Current Biology 19 (2): R81–R88. doi:10.1016/j.cub.2008.11.067. PMID 19174147. https://linkinghub.elsevier.com/retrieve/pii/S0960982208014851.
- ↑ Clark, David P.; Pazdernik, Nanette J. (2013), "Molecular Evolution" (in en), Molecular Biology (Elsevier): pp. 812–853, doi:10.1016/b978-0-12-378594-7.00026-3, ISBN 978-0-12-378594-7, https://linkinghub.elsevier.com/retrieve/pii/B9780123785947000263, retrieved 2022-03-27
- ↑ Nowack, Eva C.M.; Weber, Andreas P.M. (2018-04-29). "Genomics-Informed Insights into Endosymbiotic Organelle Evolution in Photosynthetic Eukaryotes" (in en). Annual Review of Plant Biology 69 (1): 51–84. doi:10.1146/annurev-arplant-042817-040209. ISSN 1543-5008. https://www.annualreviews.org/doi/10.1146/annurev-arplant-042817-040209.
- ↑ Adl, Sina M.; Simpson, Alastair G. B.; Farmer, Mark A.; Andersen, Robert A.; Anderson, O. Roger; Barta, John R.; Bowser, Samuel S.; Brugerolle, Guy et al. (October 2005). "The New Higher Level Classification of Eukaryotes with Emphasis on the Taxonomy of Protists" (in en). The Journal of Eukaryotic Microbiology 52 (5): 399–451. doi:10.1111/j.1550-7408.2005.00053.x. ISSN 1066-5234. PMID 16248873. https://onlinelibrary.wiley.com/doi/10.1111/j.1550-7408.2005.00053.x.
- ↑ Lim, Liting; McFadden, Geoffrey Ian (2010-03-12). "The evolution, metabolism and functions of the apicoplast" (in en). Philosophical Transactions of the Royal Society B: Biological Sciences 365 (1541): 749–763. doi:10.1098/rstb.2009.0273. ISSN 0962-8436. PMID 20124342.
- ↑ Knoll, Andrew H. (July 2015). "Paleobiological Perspectives on Early Microbial Evolution" (in en). Cold Spring Harbor Perspectives in Biology 7 (7): a018093. doi:10.1101/cshperspect.a018093. ISSN 1943-0264. PMID 26134315.
- ↑ Javaux, Emmanuelle J.; Marshall, Craig P.; Bekker, Andrey (February 2010). "Organic-walled microfossils in 3.2-billion-year-old shallow-marine siliciclastic deposits" (in en). Nature 463 (7283): 934–938. doi:10.1038/nature08793. ISSN 0028-0836. PMID 20139963. Bibcode: 2010Natur.463..934J. http://www.nature.com/articles/nature08793.
- ↑ Knoll, A.H; Javaux, E.J; Hewitt, D; Cohen, P (2006-06-29). "Eukaryotic organisms in Proterozoic oceans" (in en). Philosophical Transactions of the Royal Society B: Biological Sciences 361 (1470): 1023–1038. doi:10.1098/rstb.2006.1843. ISSN 0962-8436. PMID 16754612.
- ↑ Parfrey, Laura Wegener; Lahr, Daniel J. G.; Knoll, Andrew H.; Katz, Laura A. (2011-08-16). "Estimating the timing of early eukaryotic diversification with multigene molecular clocks" (in en). Proceedings of the National Academy of Sciences 108 (33): 13624–13629. doi:10.1073/pnas.1110633108. ISSN 0027-8424. PMID 21810989. Bibcode: 2011PNAS..10813624P.
- ↑ Douzery, Emmanuel J. P.; Snell, Elizabeth A.; Bapteste, Eric; Delsuc, Frédéric; Philippe, Hervé (2004-10-26). "The timing of eukaryotic evolution: Does a relaxed molecular clock reconcile proteins and fossils?" (in en). Proceedings of the National Academy of Sciences 101 (43): 15386–15391. doi:10.1073/pnas.0403984101. ISSN 0027-8424. PMID 15494441. Bibcode: 2004PNAS..10115386D.
- ↑ Cavalier-Smith, T. (March 1987). "Eukaryotes with no mitochondria" (in en). Nature 326 (6111): 332–333. doi:10.1038/326332a0. ISSN 0028-0836. PMID 3561476. Bibcode: 1987Natur.326..332C. http://www.nature.com/articles/326332a0.
- ↑ Poole, Anthony; Penny, David (June 2007). "Engulfed by speculation" (in en). Nature 447 (7147): 913. doi:10.1038/447913a. ISSN 0028-0836. PMID 17581566. http://www.nature.com/articles/447913a.
- ↑ Pisani, Davide; Cotton, James A.; McInerney, James O. (August 2007). "Supertrees Disentangle the Chimerical Origin of Eukaryotic Genomes" (in en). Molecular Biology and Evolution 24 (8): 1752–1760. doi:10.1093/molbev/msm095. ISSN 1537-1719. PMID 17504772. https://academic.oup.com/mbe/article-lookup/doi/10.1093/molbev/msm095.
- ↑ López-García, Purificación; Moreira, David (May 2006). "Selective forces for the origin of the eukaryotic nucleus" (in en). BioEssays 28 (5): 525–533. doi:10.1002/bies.20413. ISSN 0265-9247. PMID 16615090. https://onlinelibrary.wiley.com/doi/10.1002/bies.20413.
- ↑ Pittis, Alexandros A.; Gabaldón, Toni (March 2016). "Late acquisition of mitochondria by a host with chimaeric prokaryotic ancestry" (in en). Nature 531 (7592): 101–104. doi:10.1038/nature16941. ISSN 0028-0836. PMID 26840490. Bibcode: 2016Natur.531..101P.