Biology:ZNF821
 Generic protein structure example |
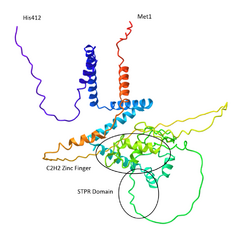
Zinc Finger Protein 821, also known as ZNF821, is a protein encoded by the ZNF821 gene. This gene is located on the 16th chromosome and is expressed highly in the testes, moderately expressed in the brain and low expression in 23 other tissues. The protein encoded is 412 amino acids long with 2 Zinc Finger motifs (C2H2 type) and a 23 amino acid long STPR domain.
Gene
Locus
ZNF821 is located at 16q22.2 on the minus strand, it is composed of 35,657 bases spanning from base 71,893,583 to 71,929,239. ZNF821 has 8 exons and is located in the same neighborhood as 4 other genes, ATXNL1, IST1, PKD1L3, AP1G1.[1]
Transcriptional Regulation
Transcription of ZNF821 is handled by the promoter GXP_9784938 which is 539 bases long and located from base 71,884,046 to 71,884,585. The promoter region begins 404 base pairs upstream of the beginning of transcription. Several transcription factors with scores greater than 0.9 are predicted to regulate ZNF821 expression.
| Transcription Factor | Abbreviation | Binding Site | Strand |
|---|---|---|---|
| Myeloid zinc finger protein | MZF1 | GTGGGGATCCG | + |
| Cyclin D binding myb-like transcription factor | DMTF | CACCCCTAGGCCCGA | - |
| Early growth response 2 | EGRF | GAGAGGGGGTGCCTGCGGC | + |
| Human acute myelogenous leukemia factors | HAML | AGCTGTGGTTGGGGG | + |
| C2H2 zinc finger transcription factors 2 | ZF02 | GACTTGAGCTACCACCCCATTCT | - |
| Hypoxia-response elements | HIFF | CCATCCCACCGCAAATGTGCAGGTC | - |
| Ets variant 1 | ETSF | CACGTCCAGGAAGGTCTGGGG | + |
| Homeobox transcription factor Nanog | HOXF | ACCCGGGAATGGGCGAGGC | + |
| GLIS family zinc finger 3 | GLIF | CGCTCCGCCCCCCAAGG | - |
| GTF2I-like repeat 4 of GTF3 | GUCE | CGGGATTGGGC | + |
| zinc finger protein with KRAB and SCAN domains 12 | ZF07 | GGAGCCCCTCCTCTCCA | + |
| Myc associated zinc finger protein | MAZF | GTCTCGGGGAGAGGAGTCCGGGGCGGGTGTT | - |
| Zinc finger and BTB domain containing 14 | VF5F | CGGTCCGCGCGCGGCCC | + , - |
| Transcription factor II B (TFIIB) recognition element | TF2B | CCGCGCC | - |
| Zinc finger protein 37 alpha | ZF37 | CCTCCCCCT | - |
| E2F transcription factor 1 | E2FF | CGCGCGAGGGCGGCGGG | - |
| Cas-interacting zinc finger | CIZF | GTAGAAAAAGG | - |
| Sma- and Mad-related proteins | SMAD | TCTGTCTGTCT | + |
| SRY (sex determining region Y)-box 6 | SORY | CAGACAGACAGACGACAACCGAAACAGGCAG | - |
Expression
ZNF821 is highly expressed in the testes, almost 2.5 times as much as in the brain, the next most highly expressed in tissue. Expression in the brain is primarily during fetal development, with lower levels of expression occurring in the cerebellum. There are low levels of expression in most other tissues.
mRNA
Variants and Isoforms
ZNF821 has 7 different transcript variants and 4 isoforms.[2] Variant 1 Isoform 1 is the second longest and but most abundant of all the variants and isoforms. While variant 2 is longer, it contains one fewer exon. Variant 1, Isoform 1 is 1987 bases long with a 5' UTR 415 bases long and a 3' UTR 433 bases long.
| Variant | Isoform | Length | # of Exons |
|---|---|---|---|
| 1 | 1 | 1987 | 8 |
| 2 | 1 | 2005 | 7 |
| 3 | 2 | 1894 | 7 |
| 4 | 2 | 1879 | 6 |
| 5 | 3 | 1853 | 7 |
| 6 | 4 | 1959 | 8 |
| 7 | 4 | 1722 | 7 |
Protein
Characteristics
The protein encoded by the ZNF821 gene is 412 amino acids long with a calculated molecular weight of ~ 47 kDa and a predicted isoelectric point of 6.14. Compared to the rest of the human proteome, there are decreased amounts of Isoleucine and Tyrosine residues as well as increased levels of Arginine residues.[3]
Structure
ZNF821 protein contains two C2H2 Zinc Finger motifs (spanning amino acids 120-140 and 152–172, respectively) and an STPR (one-score-and-three-amino acid peptide repeat) domain (spanning amino acids 223–314) containing a bipartite nuclear localization signal. This STPR domain is a double-stranded DNA-binding domain with similar traits to the silkworm FMBP-1 STPR domain and is thought to be responsible for the nuclear localization of the ZNF821 protein.[4] The secondary structure of the ZNF821 protein is composed of several alpha helical structures along with two small regions of beta sheets.[5][6][7][8] The tertiary structure of the ZNF821 protein provides exposure of the Zinc Fingers for presumed DNA-binding.[9]
Cellular Localization
The ZNF821 protein binds to DNA making it highly likely to be localized to the nucleus, there is also a bipartite nuclear localization sequence from Lys280 to Arg297, Lys304 to Leu320, and Lys338 to Arg354. An analysis of the subcellular localization in both close and distant orthologs resulted in a >99% chance of being localized to the nucleus for all orthologs.[10]
Regulation
The ZNF821 protein is predicted to be modified post-translationally at several different positions. When compared with both close and distant orthologous sequences two phosphorylation sites are conserved, the Serine at position 2 and the Threonine at position 7.[11] It is also predicted by several sources to have further phosphorylation sites of the Serine at position 254 and the Tyrosine at position 279.[12][11]
Interactions
Several proteins have been shown to interact with the ZNF821 protein, many of them relating to transcriptional regulation.[13]
| Abbreviation | Protein Name | Identification Method | Function |
|---|---|---|---|
| ATM | ATM Serine/Threonine kinase | Two Hybrid Array | Activates checkpoint signaling upon sensing DNA damage |
| CCDC85B | Coiled-coil domain-containing protein 85B | Two Hybrid Pooling | Transcriptional Repressor |
| SMARCA2 | Probable global transcription activator SNF2L2 | Two Hybrid Pooling | Transcriptional activation and repression by chromatin remodeling |
| CDCA7L | Cell division cycle-associated 7-like protein | Two Hybrid Array | Transcriptional Repressor |
| PIM2 | Serine/threonine-protein kinase Pim-2 | Two Hybrid Array | Proto-oncogene |
| DVL3 | Segment polarity protein dishevelled homolog DVL-3 | Two Hybrid Array | Cell signal transduction |
| RUNDC3A | RUN domain-containing protein 3A | Two Hybrid Array | Effector of RAPA2A |
| FXR1 | Fragile X mental retardation syndrome-related protein 1 | Two Hybrid Pooling | RNA-binding protein |
Homology
Paralogs
ZNF821 has no paralogs in humans.[1]
Orthologs
There are orthologs for ZNF821 across vertebrates, but none for the protein in invertebrates. The Zinc Finger motifs are conserved into invertebrates. The STPR domain is only present in mammals.
| Genus | Species | Common Name | Taxonomy | Date of Divergence (MYA) | Sequence Length (AA) | Sequence Identity (%) |
|---|---|---|---|---|---|---|
| Homo | sapiens | Human | Primates | 0.00 | 412 | 100.00 |
| Pongo | abelii | Sumatran Orangutan | Primates | 15.20 | 412 | 99.76 |
| Lacerta | agilis | Sand lizard | Squamata | 318.00 | 429 | 76.33 |
| Chrysemys | picta bellii | Western Painted turtle | Testudines | 318.00 | 411 | 87.65 |
| Gopherus | evgoodei | Sinaloan desert tortoise | Testudines | 318.00 | 413 | 86.44 |
| Antrostomus | carolinensis | Chuck-will's-widow | Caprimulgiformes | 318.00 | 411 | 86.20 |
| Apteryx | rowi | Ōkārito kiwi | Apterygiformes | 318.00 | 482 | 87.41 |
| Leptosomus | discolor | Cuckoo roller | Leptosomiformes | 318.00 | 410 | 85.68 |
| Xenopus | laevis | Two-lined caecilian | Gymnophiona | 351.70 | 409 | 76.76 |
| Geotrypetes | seraphini | Gaboon caecilian | Gymnophiona | 351.70 | 378 | 75.26 |
| Bufo | bufo | Common toad | Anura | 351.70 | 395 | 66.75 |
| Rhinatrema | bivittatum | African clawed frog | Anura | 351.70 | 398 | 69.64 |
| Pygocentrus | nattereri | Red-bellied piranha | Characiformes | 433.00 | 456 | 48.58 |
| Cyprinus | carpio | Common Carp | Cypriniformes | 433.00 | 441 | 49.56 |
| Polypterus | senegalus | Senegal bichir | Polypteriformes | 433.00 | 539 | 54.59 |
| Erpetoichthys | calabaricus | Reedfish | Polypteriformes | 433.00 | 471 | 49.58 |
| Paramormyrops | kingsleyae | Old Calabar mormyrid | Osteoglossiformes | 433.00 | 443 | 53.63 |
| Carcharodon | carcharias | Great white shark | Lamniformes | 465.00 | 360 | 42.07 |
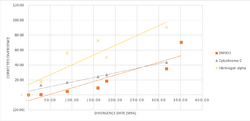
The relative rate of divergence is slow when compared to the rates of two reference proteins, Cytochrome c and Fibrinogen alpha , but increases to slightly faster than Cytochrome c as the date of divergence gets closer to the present.
Clinical Significance
ZNF821 has been associated in some capacity with several different diseases and conditions. It has been implicated in causing craniosynostosis through interactions with the transcription factor BCL11B by affecting the charges on the arginine-3, arginine-5, and lysine-3 residues, thereby increasing their conformational flexibility.[14] It has also been found to be a possible biomarker for methamphetamine-associated psychosis (MAP) via the process of RNA-degradation.[15] Another disease association is that with breast cancer as part of a DNA-repair sub network. ZNF821 was found to be dysregulated among breast cancer patients.[16] Finally, there is a study showing an increase in methylation over time on ZNF821 in Parkinson's disease patients who did not receive L-dopa/entacapone. This provides a clearer view of changes due only to Parkinson's pathophysiology.[17]
References
- ↑ 1.0 1.1 "Human BLAT Search". https://genome.ucsc.edu/cgi-bin/hgBlat.
- ↑ "ZNF821 zinc finger protein 821 [Homo sapiens (human) - Gene - NCBI"]. https://www.ncbi.nlm.nih.gov/gene/55565.
- ↑ "SAPS < Sequence Statistics < EMBL-EBI". https://www.ebi.ac.uk/Tools/seqstats/saps/.
- ↑ "STPR, a 23-amino acid tandem repeat domain, found in the human function-unknown protein ZNF821". Biochemistry 49 (38): 8367–8375. September 2010. doi:10.1021/bi100448f. PMID 20795678.
- ↑ "NPS@ : SOPMA secondary structure prediction". https://npsa-prabi.ibcp.fr/cgi-bin/npsa_automat.pl?page=/NPSA/npsa_sopma.html.
- ↑ Figure S6: Predicted secondary structure of CoV-RMEN using CFSSP:Chou and Fasman secondary structure prediction server. doi:10.7717/peerj.9572/supp-13.
- ↑ "Bioinformatics Toolkit". https://toolkit.tuebingen.mpg.de/tools/ali2d.
- ↑ "Prediction of the Secondary Structure by GOR". http://cib.cf.ocha.ac.jp/bitool/GOR/.
- ↑ "AlphaFold Protein Structure Database". https://alphafold.ebi.ac.uk/.
- ↑ "Services" (in en). https://services.healthtech.dtu.dk/.
- ↑ 11.0 11.1 "Motif Scan" (in en). https://myhits.sib.swiss/cgi-bin/motif_scan.
- ↑ "PhosphoSitePlus". https://www.phosphosite.org/homeAction.
- ↑ "PSICQUIC View". http://www.ebi.ac.uk/Tools/webservices/psicquic/view/home.xhtml;jsessionid=73BD9A8D5B30A4700F34B1BF0138F9CB?conversationContext=1.
- ↑ "A de novo substitution in BCL11B leads to loss of interaction with transcriptional complexes and craniosynostosis". Human Molecular Genetics 28 (15): 2501–2513. August 2019. doi:10.1093/hmg/ddz072. PMID 31067316.
- ↑ "Candidate gene networks and blood biomarkers of methamphetamine-associated psychosis: an integrative RNA-sequencing report". Translational Psychiatry 6 (5): e802. May 2016. doi:10.1038/tp.2016.67. PMID 27163203.
- ↑ "Systematic identification of molecular links between core and candidate genes in breast cancer". Journal of Molecular Biology 427 (6 Pt B): 1436–1450. March 2015. doi:10.1016/j.jmb.2015.01.014. PMID 25640309.
- ↑ "DNA methylation changes associated with Parkinson's disease progression: outcomes from the first longitudinal genome-wide methylation analysis in blood". Epigenetics 14 (4): 365–382. April 2019. doi:10.1080/15592294.2019.1588682. PMID 30871403.
 |