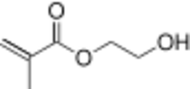
Chemistry:(Hydroxyethyl)methacrylate
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Hydroxyethyl 2-methylprop-2-enoate | |
| Other names
HEMA; hydroxyethylmethacrylate; glycol methacrylate; glycol monomethacrylate; hydroxyethyl methacrylate; ethylene glycol methacrylate; 2-(methacryloyloxy)ethanol
| |
| Identifiers | |
3D model (JSmol)
|
|
| 1071583 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| 936557 | |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| C6H10O3 | |
| Molar mass | 130.143 g·mol−1 |
| Appearance | Colourless liquid |
| Density | 1.07 g/cm3 |
| Melting point | −99 °C (−146 °F; 174 K)[2] |
| Boiling point | 213 °C (415 °F; 486 K)[2] |
| miscible | |
| log P | 0.50[1] |
| Vapor pressure | 0.08 hPa |
| Hazards | |
| Main hazards | Eye irritation |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H317, H319 | |
| P261, P264, P272, P280, P302+352, P305+351+338, P321, P332+313, P333+313, P337+313, P362, P363, P501 | |
| Flash point | 97 °C (207 °F; 370 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Hydroxyethylmethacrylate (also known as glycol methacrylate)[3][4] is the organic compound with the chemical formula H2C\dC(CH3)CO2CH2CH2OH. It is a colorless viscous liquid that readily polymerizes. Hydroxyethylmethacrylate is a monomer that is used to make various polymers.
Synthesis
Hydroxyethylmethacrylate was first synthesized around 1925. Common methods of synthesis are:[5]
- reaction of methacrylic acid with ethylene oxide;
- esterification of methacrylic acid with a large excess of ethylene glycol.
Both these methods give also some amount of ethylene glycol dimethacrylate. During polymerization of hydroxyethylmethacrylate, it works as crosslinking agent.[5]
Properties
Hydroxyethylmethacrylate is completely miscible with water and ethanol, but its polymer is practically insoluble in common solvents. Its viscosity is 0.0701 Pa⋅s at 20°C[6] and 0.005 Pa⋅s at 30°C.[3] During polymerization, it shrinks by approximately 6%.[6]
Applications
Contact lenses
In 1960, O. Wichterle and D. Lím[7] described its use in synthesis of hydrophilic crosslinked networks, and these results had great importance for manufacture of soft contact lenses.[5] Polyhydroxyethylmethacrylate is hydrophilic: it is capable of absorbing from 10 to 600% water relative to the dry weight. Because of this property, it was one of the first materials to be used in the manufacture of soft contact lenses.[8]
Use in 3D printing
Hydroxyethylmethacrylate lends itself well to applications in 3D printing as it cures quickly at room temperature when exposed to UV light in the presence of photoinitiators. It may be used as a monomeric matrix in which 40nm silica particles are suspended for 3D glass printing.[9] When combined with a suitable blowing agent such as BOC anhydride it forms a foaming resin which expands when heated.[10]
Other
In electron microscopy, later in light microscopy, hydroxyethylmethacrylate serves as an embedding medium.[4][3]
When treated with polyisocyanates, polyhydroxyethylmethacrylate makes a crosslinked polymer, an acrylic resin, that is a useful component in some paints.[11]
Hazards
Hydroxyethylmethacrylate is a mild skin irritant and can cause allergic skin reactions.[3]
References
- ↑ "2-hydroxyethyl methacrylate_msds". ChemSrc: A Smart Chem-Search Engine. https://www.chemsrc.com/en/cas/868-77-9_1092316.html.
- ↑ 2.0 2.1 "GPS Safety Summary 2-Hydroxyethyl methacrylate (HEMA)". July 2013. http://corporate.evonik.com/_layouts/Websites/Internet/DownloadCenterFileHandler.ashx?fileid=1091.
- ↑ 3.0 3.1 3.2 3.3 Gerrits, P. O.; Horobin, R. W. (1996). "Glycol Methacrylate Embedding for Light Microscopy: Basic Principles and Trouble-Shooting". Journal of Histotechnology 19 (4): 297–311. doi:10.1179/his.1996.19.4.297.
- ↑ 4.0 4.1 Cole, Madison B.; Sykes, Stephen M. (1974). "Glycol Methacrylate in Light Microscopy a Routine Method for Embedding and Sectioning Animal Tissues" (in en). Stain Technology 49 (6): 387–400. doi:10.3109/10520297409117016. ISSN 0038-9153. PMID 4142140. http://www.tandfonline.com/doi/full/10.3109/10520297409117016.
- ↑ 5.0 5.1 5.2 Macret, M.; Hild, G. (1982). "Hydroxyalkyl methacrylates: Kinetic investigations of radical polymerizations of pure 2-hydroxyethyl methacrylate and 2, 3-dihydroxypropyl methacrylate and the radical copolymerization of their mixtures". Polymer 23 (1): 81–90. doi:10.1016/0032-3861(82)90020-9.
- ↑ 6.0 6.1 Rosenberg, M.; Bartl, P.; Lesko, J. (1960). "Water-soluble methacrylate as an embedding medium for the preparation of ultrathin sections". Journal of Ultrastructure Research 4 (3–4): 298–303. doi:10.1016/s0022-5320(60)80024-x. PMID 13743397.
- ↑ Wichterle, O.; Lím, D. (1960). "Hydrophilic gels for biological use". Nature 185 (4706): 117–118. doi:10.1038/185117a0. Bibcode: 1960Natur.185..117W.
- ↑ Blasco, Joe; Kehoe, Vincent J-R; The professional make-up artist : motion pictures, television, print, theatre; Script error: No such module "CS1 identifiers".; LCC# PN2068.B53 2005
- ↑ Kotz, Frederik; Arnold, Karl; Bauer, Werner; Schild, Dieter; Keller, Nico; Sachsenheimer, Kai; Nargang, Tobias M.; Richter, Christiane et al. (2017). "Three-dimensional printing of transparent fused silica glass" (in en). Nature 544 (7650): 337–339. doi:10.1038/nature22061. ISSN 0028-0836. PMID 28425999.
- ↑ Wirth, D. (2020). "Highly Expandable Foam for Lithographic 3D Printing". ACS Applied Materials and Interfaces 12 (16): 19033–19043. doi:10.1021/acsami.0c02683. PMID 32267677.
- ↑ Stoye, D.; Funke, W.; Hoppe, L.; Hasselkus, J. R.; Curtis, L. G.; Hoehne, K.; Zech, H. J.; Heiling, P. et al. (2006). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a18_359.pub2.
 |
