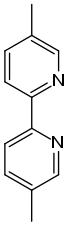
Chemistry:Abametapir
 | |
| Clinical data | |
|---|---|
| Trade names | Xeglyze |
| Other names | Ha44 |
| AHFS/Drugs.com | Professional Drug Facts |
| License data |
|
| Routes of administration | Topical |
| Drug class | Pediculicide, metalloproteinase inhibitor |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 91.3–92.3% |
| Metabolism | CYP1A2 |
| Metabolites | Hydroxyl and carboxyl derivatives |
| Elimination half-life | 21 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
| Formula | C12H12N2 |
| Molar mass | 184.242 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Abametapir, sold under the brand name Xeglyze, is a medication used for the treatment of head lice infestation in people six months of age and older.[1][2]
The most common side effects include skin redness, rash, skin burning sensation, skin inflammation, vomiting, eye irritation, skin itching, and hair color changes.[2]
Abametapir is a metalloproteinase inhibitor.[1] Abametapir was approved for medical use in the United States in July 2020.[1][3] The U.S. Food and Drug Administration (FDA) considers it to be a first-in-class medication.[4]
Medical uses
Abametapir is indicated for the topical treatment of head lice infestation in people six months of age and older.[1][2]
Contraindications
Abametapir has no contraindications according to the labeling.[5]
Adverse effects
Common adverse effects are burning skin sensations (in 3% of patients), contact dermatitis (2%), skin redness (4%), rash (3%), and vomiting (2%).[5]
Interactions
Abametapir blocks the liver enzymes CYP3A4, CYP2B6 and CYP1A2 in vitro. A single application of the drug may lead to increased blood concentrations of drugs that are metabolized by these enzymes.[1]
Pharmacology
Mechanism of action
The drug inhibits enzymes called metalloproteinases. In lice, these enzymes play a role in egg development and survival;[1] and consequently, blocking them will disrupt the lice's life cycle.
Pharmacokinetics
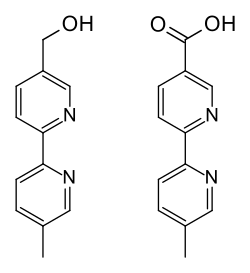
After application to the scalp, part of the substance reaches the bloodstream, where most of it (91.3–92.3%) is bound to plasma proteins. It is metabolized primarily by the liver enzyme CYP1A2 to abametapir hydroxyl and further to abametapir carboxyl (see structure drawings). Abametapir carboxyl has a plasma protein binding of 96.0–97.5% and is the predominant of the three substances in the circulation, having a Cmax 30 times and an area under the curve (AUC) 250 times that of abametapir itself.[1]
The elimination half-life of abametapir is 21 hours. That of abametapir carboxyl is not well known; it is thought to be 71±40 hours or longer. It is not known whether the drug is eliminated via the urine or the faeces.[1]
History
The U.S. Food and Drug Administration (FDA) approved abametapir based on evidence from two identical clinical trials of 699 participants with head lice.[2] The trials were conducted at fourteen sites in the United States.[2]
The benefit and side effects of abametapir were evaluated in two clinical trials that enrolled participants with head lice who were at least six months old.[2]
About half of all enrolled participants was randomly assigned to abametapir and the other half to placebo.[2] Abametapir lotion or placebo lotion were applied once as a ten-minute treatment to infested hair.[2] The benefit of abametapir in comparison to placebo was assessed after 1, 7 and 14 days by comparing the counts of participants in each group who were free of live lice.[2]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 "Xeglyze (abametapir) lotion, for topical use". Dr. Reddy's Laboratories. Inc.. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/206966lbl.pdf.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 "Drug Trial Snapshot: Xeglyze". 24 July 2020. https://www.fda.gov/drugs/drug-approvals-and-databases/drug-trial-snapshot-xeglyze.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Drug Approval Package: Xeglyze". 21 August 2020. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/206966Orig1s000TOC.cfm.
- ↑ "New Drug Therapy Approvals 2020". 31 December 2020. https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/new-drug-therapy-approvals-2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 5.0 5.1 Abametapir Professional Drug Facts. Accessed 6 May 2021.
Further reading
- "Clinical studies evaluating abametapir lotion, 0.74%, for the treatment of head louse infestation". Pediatr Dermatol 35 (5): 616–621. September 2018. doi:10.1111/pde.13612. PMID 29999197.
External links
- "Abametapir". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/abametapir.
 |

