Chemistry:Aluminium lactate
From HandWiki
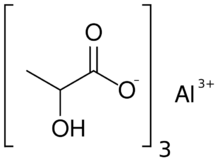
| |
| Names | |
|---|---|
| Other names
Aluminium trilactate, tris(2-hydroxypropanoato)aluminium
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| C9H15AlO9 | |
| Molar mass | 294.192 g·mol−1 |
| Appearance | White powder |
| Melting point | 300 °C (572 °F; 573 K) |
| Soluble | |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P302, P352, P305, P351, P338 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Aluminium lactate is a chemical compound, a salt of aluminium and lactic acid with the formula Al(C3H5O3)3.[1][2]
Synthesis
Aluminium lactate is obtained by precipitating a solution of the barium salt by aluminium sulfate.[3]
Physical properties
Aluminium lactate appears as a white powder which is soluble in water.
Use
Aluminium lactate is used as a mordant.[4]
It is suitable for use in both the cosmetic[5] and oral industries.[6][7]
Aluminium lactate is also used as a precursor for sol–gel synthesis of alumina-based glasses.[8]
References
- ↑ Vargel, Christian (12 May 2020) (in en). Corrosion of Aluminium. Elsevier. p. 748. ISBN 978-0-08-099927-2. https://books.google.com/books?id=E-p9BAAAQBAJ&dq=aluminium+lactate&pg=PA748. Retrieved 24 January 2022.
- ↑ "Aluminum L-lactate". American Elements. https://www.americanelements.com/aluminum-l-lactate-18917-91-4.
- ↑ (in en) Proceedings of the American Pharmaceutical Association at the Annual Meeting. American Pharmaceutical Association. 1887. p. 291. https://books.google.com/books?id=4m0CAAAAYAAJ&dq=aluminium+lactate&pg=PA291. Retrieved 24 January 2022.
- ↑ "Aluminium Lactate - mordant for natural dyeing plant (cellulose) fibres". DT Craft and Design. 14 August 2021. https://www.dtcrafts.co.uk/shop/aluminium-lactate-mordant-natural-dyeing-plant-cellulose-fibres/.
- ↑ Hunt, Laura; Tankeu, Raissa; Thilk, Alexia; Coppenrath, Valerie (2014). "Ammonium Lactate–Containing Moisturizers: A Systematic Review" (in en). U.S. Pharmacist 39 (11): 46–49. https://www.uspharmacist.com/article/ammonium-lactatecontaining-moisturizers-a-systematic-review. Retrieved 24 January 2022.
- ↑ "Aluminium Lactate by DPL-US - Personal Care & Cosmetics". ulprospector.com. https://www.ulprospector.com/en/na/PersonalCare/Detail/10730/329300/Aluminium-Lactate.
- ↑ Lussi, Adrian (1 January 2006) (in en). Dental Erosion: From Diagnosis to Therapy. Karger Medical and Scientific Publishers. p. 182. ISBN 978-3-8055-8097-7. https://books.google.com/books?id=-l0ROzorSREC&dq=aluminium+lactate&pg=PA182. Retrieved 24 January 2022.
- ↑ Zhang, Long; de Araujo, Carla C.; Eckert, Hellmut (May 2007). "Aluminum lactate – An attractive precursor for sol–gel synthesis of alumina-based glasses". Journal of Non-Crystalline Solids 353 (13–15): 1255–1260. doi:10.1016/j.jnoncrysol.2006.10.065. Bibcode: 2007JNCS..353.1255Z. https://www.researchgate.net/publication/243302120.
 |

