Chemistry:Arsthinol
From HandWiki
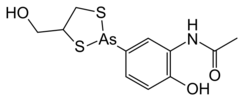
| |
| Names | |
|---|---|
| Preferred IUPAC name
N-{2-Hydroxy-5-[4-(hydroxymethyl)-1,3,2-dithiarsolan-2-yl]phenyl}acetamide | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C11H14AsNO3S2 | |
| Molar mass | 347.28 g·mol−1 |
| Pharmacology | |
| 1=ATC code }} | P01AR01 (WHO) QP51AD01 (WHO) |
| Oral | |
| Pharmacokinetics: | |
| 89 % Hepatic[1] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Arsthinol (INN) is an antiprotozoal agent. It was synthesized for the first time in 1949 by Ernst A.H. Friedheim by complexation of acetarsol with 2,3-dimercaptopropanol (British anti-Lewisite)[2] and has been demonstrated to be effective against amoebiasis and yaws. It was marketed few years later by Endo Products (Balarsen, Tablets, 0.1 g).[3] Among trivalent organoarsenicals, arsthinol was considered as very well tolerated.[4] Recently, it was studied for its anticancer activity.[5][6]
References
- ↑ Cristau, B; Chabas, ME; Placidi, M (1975). "Voies et cinétiques d'excrétion de l'arsenic chez le Cobaye après injection de divers médicaments organo-arséniés". Ann Pharm Fr 33: 577–89.
- ↑ Friedheim, Ernst AH (1949). "A Five Day Peroral Treatment of Yaws with STB, a New Trivalent Arsenical". Am J Trop Med Hyg s1-29 (2): 185–188. doi:10.4269/ajtmh.1949.s1-29.185. PMID 18116845.
- ↑ Anonyme (1953). "New and nonofficial remedies; arsthinol". J Am Med Assoc 152: 531.
- ↑ Brown, CH; Gebhart, WF; Reich, A (1956). "Intestinal amebiasis: incidence, symptoms, and treatment with arsthinol (Balarsen)". JAMA 160 (5): 360–363. doi:10.1001/jama.1956.02960400018005. PMID 13278204.
- ↑ Gibaud, SExpression error: Unrecognized word "et". (2006). "(2-Phenyl-[1, 3, 2] dithiarsolan-4-yl)-methanol derivatives show in vitro antileukemic activity". J Organomet Chem 691 (5): 1081–1084. doi:10.1016/j.jorganchem.2005.11.007.
- ↑ Becherirat, S.; Lanhers, M.-C.; Socha, M.; Yemloul, M.; Astier, A.; Loboda, C.; Aniceto, N.; Gibaud, S. (2013). "The antitumor effects of an arsthinol-cyclodextrin complex in an heterotopic mouse model of glioma". Eur J Pharm Biopharm 85 (3): 560–568. doi:10.1016/j.ejpb.2013.06.021. PMID 23831266. https://hal.archives-ouvertes.fr/hal-01169157/file/Eur%20J%20Pharm%20Biopharm%202013%20Becherirat.pdf.
 |

