Chemistry:Fumagillin
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
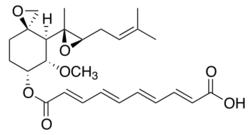
| Formula | C26H34O7 |
| Molar mass | 458.551 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Fumagillin is a complex biomolecule and used as an antimicrobial agent. It was isolated in 1949 from the microbial organism Aspergillus fumigatus.[1]
Uses
In animals
It was originally used against microsporidian parasites Nosema apis infections in honey bees.[citation needed]
Some studies found it to be effective against some myxozoan parasites, including Myxobolus cerebralis, an important parasite of fish; however, in the more rigorous tests required for U.S. Food and Drug Administration approval, it was ineffective.[citation needed]
There are reports that fumagillin controls Nosema ceranae,[2] which has recently been hypothesized as a possible cause of colony collapse disorder.[3][4] The latest report, however, has shown it to be ineffective against N. ceranae.[5] Fumagillin is also investigated as an inhibitor of malaria parasite growth.[6][7]
In humans
Fumagillin has been used in the treatment of microsporidiosis.[8][9] It is also an amebicide.[10]
Fumagillin can block blood vessel formation by binding to an enzyme methionine aminopeptidase 2[11] and for this reason, the compound, together with semisynthetic derivatives, are investigated as an angiogenesis inhibitor[12] in the treatment of cancer.
The company Zafgen conducted clinical trials using the fumagillin analog beloranib for weight loss,[13] but they were unsuccessful.[14]
Fumagillin is toxic to erythrocytes in vitro at concentrations greater than 10 μM.[15]
Total synthesis
Fumagillin and the related fumagillol (the hydrolysis product) have been a target in total synthesis, with several reported successful strategies, racemic, asymmetric, and formal.[16][17][18][19][20][21][22][23][24]
References
- ↑ F. R. Hanson, T. E. Elbe, J. Bacteriol. 1949, 58, 527
- ↑ "Does fumagillin control the recently detected invasive parasite Nosema ceranae in western honey bees (Apis mellifera)?". Journal of Invertebrate Pathology 99 (3): 342–344. November 2008. doi:10.1016/j.jip.2008.04.005. PMID 18550078.
- ↑ Sabin Russell (2007-04-26). "UCSF scientist tracks down suspect in honeybee deaths". San Francisco Chronicle. http://www.sfgate.com/cgi-bin/article.cgi?file=/c/a/2007/04/26/MNGK7PFOMS1.DTL.
- ↑ "Scientists Identify Pathogens That May Be Causing Global Honeybee Deaths". Edgewood Chemical Biological Center. 2007-04-25. http://www.ecbc.army.mil/pr/download/042507_honey_bee_pathogens.pdf.[verification needed]
- ↑ "Nosema ceranae escapes fumagillin control in honey bees". PLOS Pathogens 9 (3): e1003185. March 2013. doi:10.1371/journal.ppat.1003185. PMID 23505365.
- ↑ "Fumagillin and fumarranol interact with P. falciparum methionine aminopeptidase 2 and inhibit malaria parasite growth in vitro and in vivo". Chemistry & Biology 16 (2): 193–202. February 2009. doi:10.1016/j.chembiol.2009.01.006. PMID 19246010.
- ↑ Christopher Arico-Muendel et al. "Antiparasitic activities of novel, orally available fumagillin analogs". Bioorganic & Medicinal Chemistry Letters Vol. 19 Nr. 17 (2009), blz. 5128-5131 "Antiparasitic activities of novel, orally available fumagillin analogs". Bioorganic & Medicinal Chemistry Letters 19 (17): 5128–5131. September 2009. doi:10.1016/j.bmcl.2009.07.029. PMID 19648008.
- ↑ "Microsporidiosis in solid organ transplant recipients: two Enterocytozoon bieneusi cases and review". Transplant Infectious Disease 11 (1): 83–88. February 2009. doi:10.1111/j.1399-3062.2008.00347.x. PMID 18803616.
- ↑ "Fumagillin treatment of intestinal microsporidiosis". The New England Journal of Medicine 346 (25): 1963–1969. June 2002. doi:10.1056/NEJMoa012924. PMID 12075057.
- ↑ "Fumagillin: an anti-infective as a parent molecule for novel angiogenesis inhibitors". Expert Review of Anti-Infective Therapy 5 (4): 573–579. August 2007. doi:10.1586/14787210.5.4.573. PMID 17678422.
- ↑ "Whirling disease of salmonid fish: life cycle, biology, and disease". The Journal of Parasitology 89 (4): 658–667. August 2003. doi:10.1645/GE-82R. PMID 14533670.
- ↑ "Synthetic analogues of fumagillin that inhibit angiogenesis and suppress tumour growth". Nature 348 (6301): 555–557. December 1990. doi:10.1038/348555a0. PMID 1701033. Bibcode: 1990Natur.348..555I.
- ↑ "Zafgen Announces Positive Topline Phase 1b Data for ZGN-433 in Obesity". MedNews. Drugs.com. 5 January 2011. https://www.drugs.com/clinical_trials/zafgen-announces-positive-topline-phase-1b-data-zgn-433-obesity-10955.html.
- ↑ "Zafgen Halts Development of Beloranib, to Cut Jobs by ~34%". nasdaq.com. July 20, 2016. http://www.nasdaq.com/article/zafgen-halts-development-of-beloranib-to-cut-jobs-by-34-cm651992.
- ↑ "Stimulation of suicidal erythrocyte death by fumagillin". Basic & Clinical Pharmacology & Toxicology 112 (5): 346–351. May 2013. doi:10.1111/bcpt.12033. PMID 23121865.
- ↑ "A total synthesis of (+-)-fumagillin". Journal of the American Chemical Society 94 (7): 2549–2550. April 1972. doi:10.1021/ja00762a080. PMID 5016935.
- ↑ "An asymmetric total synthesis of (−)-fumagillol". Tetrahedron Letters 38 (25): 4437–4440. 1997. doi:10.1016/S0040-4039(97)00925-8.
- ↑ "A Concise Synthesis of Fumagillol". Angewandte Chemie 38 (7): 971–974. April 1999. doi:10.1002/(SICI)1521-3773(19990401)38:7<971::AID-ANIE971>3.0.CO;2-W. PMID 29711854.
- ↑ "A Concise Synthesis of Fumagillol". Synlett 2001 (5): 0661–0663. 2001. doi:10.1055/s-2001-13359.
- ↑ "Synthesis of (−)-Fumagillin". Journal of the American Chemical Society 121 (23): 5589. 1999. doi:10.1021/ja990784k.
- ↑ "A new, ring closing metathesis-based synthesis of (-)-fumagillol". Organic Letters 3 (17): 2737–2740. August 2001. doi:10.1021/ol016343z. PMID 11506622.
- ↑ "A Stereoselective Formal Synthesis of (−)-Fumagillol". European Journal of Organic Chemistry 2004 (18): 3813. 2004. doi:10.1002/ejoc.200400262.
- ↑ "Concise enantio- and diastereoselective total syntheses of fumagillol, RK-805, FR65814, ovalicin, and 5-demethylovalicin". Angewandte Chemie 45 (5): 789–793. January 2006. doi:10.1002/anie.200502826. PMID 16365904.
- ↑ "Syntheses of fumagillin and ovalicin". Chemistry 16 (13): 3884–3901. April 2010. doi:10.1002/chem.200902433. PMID 20209516.
 |
