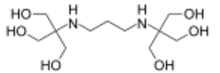
Chemistry:Bis-tris propane
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
2,2′-[Propane-1,3-diylbis(azanediyl)]bis[2-(hydroxymethyl)propane-1,3-diol] | |
| Other names
2,2'-(Propane-1,3-diyldiimino)bis[2-(hydroxymethyl)propane-1,3-diol][citation needed]
| |
| Identifiers | |
3D model (JSmol)
|
|
| 1786109 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| EC Number |
|
| 1734507 | |
| MeSH | 1,3-bis(tris(hydroxymethyl)methylamino)propane |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C11H26N2O6 | |
| Molar mass | 282.337 g·mol−1 |
| Appearance | White crystals |
| Melting point | 164 to 165 °C (327 to 329 °F; 437 to 438 K) |
| log P | −2.794 |
| Related compounds | |
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Bis-tris propane, or 1,3-bis(tris(hydroxymethyl)methylamino)propane, also known as BTP, is a chemical substance that is used in buffer solutions. It is a white to off-white crystalline powder that is soluble in water. It has a wide buffering range, from 6 to 9.5 due to its two pKa values which are close in value. This buffer is primarily used in biochemistry and molecular biology.
Applications
A review of DNA polymerase fidelity cites bis-tris propane as a suitable buffer for polymerase chain reaction (PCR).[1] Bis-Tris propane has also been used with HCl buffer for stabilization of farnesyl diphosphate isolated from a strain of Saccharomyces cerevisiae.[2] It has also been used in a study of the effects of buffer identity on electric signals of light-excited bacteriorhodopsin.[3] Use of Bis-Tris propane has also been documented in an investigation of the MgATPase activity of the myosin subfragment 1 monomer.[4] The effect of buffer identity on the kinetics of the restriction enzyme EcoRV has been studied in various buffers, including Bis-Tris propane.[5] Bis-Tris propane wide buffering range is also useful for calibration of genetically encoded pH indicators expressed in the cytosol or mitochondria.[6] Bis-Tris propane has been used as the buffering agent in separation of full and empty capsids of recombinant adeno-associated virus vectors with anion-exchange chromatography.[7][8]
See also
References
- ↑ "DNA polymerase fidelity and the polymerase chain reaction". PCR Methods and Applications 1 (1): 17–24. August 1991. doi:10.1101/gr.1.1.17. PMID 1842916.
- ↑ "Detection of farnesyl diphosphate accumulation in yeast ERG9 mutants". Analytical Biochemistry 317 (2): 180–185. June 2003. doi:10.1016/S0003-2697(03)00138-6. PMID 12758256.
- ↑ "Buffer effects on electric signals of light-excited bacteriorhodopsin". Biophysical Journal 78 (6): 3170–3177. June 2000. doi:10.1016/S0006-3495(00)76853-6. PMID 10827993. Bibcode: 2000BpJ....78.3170T.
- ↑ "MgATPase activity of myosin subfragment 1. The dimer is more active than the monomer". Journal of Molecular Biology 191 (2): 247–254. September 1986. doi:10.1016/0022-2836(86)90261-5. PMID 2949083.
- ↑ "Buffer effects on EcoRV kinetics as measured by fluorescent staining and digital imaging of plasmid cleavage". Analytical Biochemistry 268 (2): 201–212. March 1999. doi:10.1006/abio.1998.3079. PMID 10075809.
- ↑ "Mitochondrial free Ca²⁺ levels and their effects on energy metabolism in Drosophila motor nerve terminals". Biophysical Journal 104 (11): 2353–2361. June 2013. doi:10.1016/j.bpj.2013.03.064. PMID 23746507. Bibcode: 2013BpJ...104.2353I.
- ↑ "Analysis of particle content of recombinant adeno-associated virus serotype 8 vectors by ion-exchange chromatography". Human Gene Therapy Methods 23 (1): 56–64. February 2012. doi:10.1089/hgtb.2011.217. PMID 22428980.
- ↑ "Anion-exchange HPLC assay for separation and quantification of empty and full capsids in multiple adeno-associated virus serotypes" (in English). Molecular Therapy: Methods & Clinical Development 21: 548–558. June 2021. doi:10.1016/j.omtm.2021.04.003. PMID 33997103.
 |

