Chemistry:Bromoethane
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Bromoethane[2] | |||
| Other names | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 1209224 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| EC Number |
| ||
| KEGG | |||
| MeSH | bromoethane | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1891 | ||
| |||
| |||
| Properties | |||
| C2H5Br | |||
| Molar mass | 108.966 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Odor | ether-like | ||
| Density | 1.46 g mL−1 | ||
| Melting point | −120 to −116 °C; −184 to −177 °F; 153 to 157 K | ||
| Boiling point | 38.0 to 38.8 °C; 100.3 to 101.8 °F; 311.1 to 311.9 K | ||
| 1.067 g/100 mL (0 °C) 0.914 g/100 mL (20 °C) 0.896 g/100 mL (30 °C) | |||
| Solubility | miscible with ethanol, ether, chloroform, organic solvents | ||
| log P | 1.809 | ||
| Vapor pressure | 51.97 kPa (at 20 °C) | ||
Henry's law
constant (kH) |
1.3 μmol Pa−1 kg−1 | ||
| -54.70·10−6 cm3/mol | |||
Refractive index (nD)
|
1.4225 | ||
| Viscosity | 402 Pa.s (at 20 °C) | ||
| Thermochemistry | |||
Heat capacity (C)
|
105.8 J K−1 mol−1 | ||
Std enthalpy of
formation (ΔfH⦵298) |
−97.6–93.4 kJ mol−1 | ||
| Hazards | |||
| GHS pictograms |   
| ||
| GHS Signal word | DANGER | ||
| H225, H302, H332, H351 | |||
| P210, P281 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −23 °C (−9 °F; 250 K) | ||
| 511 °C (952 °F; 784 K) | |||
| Explosive limits | 6.75–11.25% | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
1.35 g kg−1 (oral, rat) | ||
LC50 (median concentration)
|
26,980 ppm (rat, 1 hr) 16,230 ppm (mouse, 1 hr) 4681 ppm (rat) 2723 ppm (mouse)[3] | ||
LCLo (lowest published)
|
3500 ppm (mouse) 24,000 ppm (guinea pig, 30 min) 7000 ppm (guinea pig, >4.5 hr)[3] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 200 ppm (890 mg/m3)[1] | ||
REL (Recommended)
|
None established[1] | ||
IDLH (Immediate danger)
|
2000 ppm[1] | ||
| Related compounds | |||
Related alkanes
|
| ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
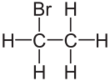
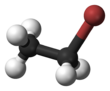
Bromoethane, also known as ethyl bromide, is a chemical compound of the haloalkanes group. It is abbreviated by chemists as EtBr (which is also used as an abbreviation for ethidium bromide). This volatile compound has an ether-like odor.
Preparation
The preparation of EtBr stands as a model for the synthesis of bromoalkanes in general. It is usually prepared by the addition of hydrogen bromide to ethene:
- H2C=CH2 + HBr → H3C-CH2Br
Bromoethane is inexpensive and would rarely be prepared in the laboratory. A laboratory synthesis includes reacting ethanol with a mixture of hydrobromic and sulfuric acids. An alternate route involves refluxing ethanol with phosphorus and bromine; phosphorus tribromide is generated in situ.[4]
Uses
In organic synthesis, EtBr is the synthetic equivalent of the ethyl carbocation (Et+) synthon.[5] In reality, such a cation is not actually formed. For example, carboxylates salts are converted to ethyl esters,[6] carbanions to ethylated derivatives, thiourea into ethylisothiouronium salts,[7] and amines into ethylamines.[8]
Safety
Short chain monohalocarbons in general are potentially dangerous alkylating agents. Bromides are better alkylating agents than chlorides, thus exposure to them should be minimized. EtBr is classified by the State of California as carcinogenic and a reproductive toxin. [citation needed]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 NIOSH Pocket Guide to Chemical Hazards. "#0265". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0265.html.
- ↑ "bromoethane - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 26 March 2005. Identification. https://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=6332&loc=ec_rcs#x291. Retrieved 15 June 2012.
- ↑ 3.0 3.1 "Ethyl bromide". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/idlh/74964.html.
- ↑ Oliver Kamm; C. S. Marvel (1941). "Alkyl and alkylene bromides". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=cv1p0025.; Collective Volume, 1, pp. 25
- ↑ Makosza, M.; Jonczyk, A.. "Phase-Transfer Alkylation of Nitriles: 2-Phenylbutyronitrile". Organic Syntheses 55: 91. http://www.orgsyn.org/demo.aspx?prep=cv6p0897.; Collective Volume, 6, pp. 897
- ↑ Petit, Y.; Larchevêque, M.. "Ethyl Glycidate from (S)-Serine: Ethyl (R)-(+)-2,3-Epoxypropanoate". Organic Syntheses 75: 37. http://www.orgsyn.org/demo.aspx?prep=v75p0037.; Collective Volume, 10, pp. 401
- ↑ E. Brand; Brand, F. C.. "Guanidodacetic Acid". Organic Syntheses 22: 440. http://www.orgsyn.org/demo.aspx?prep=cv3p0440.; Collective Volume, 3
- ↑ Brasen, W. R; Hauser, C. R.. "o-Methylethylbenzyl Alcohol". Organic Syntheses 34: 58. http://www.orgsyn.org/demo.aspx?prep=cv4p0582.; Collective Volume, 4, pp. 582
External links
- International Chemical Safety Card 1378
- NIOSH Pocket Guide to Chemical Hazards
- IARC Monograph: "Bromoethane"
 |