Chemistry:Coniferyl alcohol
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
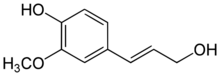
4-[(1E)-3-Hydroxyprop-1-en-1-yl]-2-methoxyphenol | |
| Other names
4-hydroxy-3-methoxycinnamyl alcohol
Coniferol | |
| Identifiers | |
3D model (JSmol)
|
|
| 2048963 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C10H12O3 | |
| Molar mass | 180.203 g·mol−1 |
| Melting point | 74 °C (165 °F; 347 K) |
| Boiling point | 163 to 165 °C (325 to 329 °F; 436 to 438 K) |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Coniferyl alcohol is an organic compound with the formula HO(CH3O)C6H3CH=CHCH2OH. A colourless or white solid, it is one of the monolignols, produced via the phenylpropanoid biochemical pathway. When copolymerized with related aromatic compounds, coniferyl alcohol forms lignin or lignans.[1][2][3] Coniferin is a glucoside of coniferyl alcohol. Coniferyl alcohol is an intermediate in biosynthesis of eugenol and of stilbenoids and coumarin. Gum benzoin contains significant amount of coniferyl alcohol and its esters. It is found in both gymnosperm and angiosperm plants. Sinapyl alcohol and paracoumaryl alcohol, the other two lignin monomers, are found in angiosperm plants and grasses.
Occurrence
Coniferyl alcohol is produced from coniferyl aldehyde by the action of dehydrogenase enzymes.[3]
It is a queen retinue pheromone (QRP), a type of honey bee pheromone found in the mandibular glands.[4]
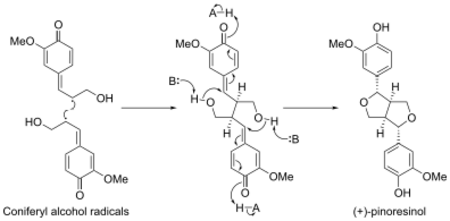
In Forsythia intermedia a dirigent protein was found to direct the stereoselective biosynthesis of (+)-pinoresinol from coniferyl alcohol.[5] Recently, a second, enantiocomplementary dirigent protein was identified in Arabidopsis thaliana, which directs enantioselective synthesis of (−)-pinoresinol.[6]
References
- ↑ Iiyama, Kenji; Lam, Thi Bach-Tuyet; Stone, Bruce A. (1994). "Covalent Cross-Links in the Cell Wall". Plant Physiology 104 (2): 315–320. doi:10.1104/pp.104.2.315. PMID 12232082.
- ↑ Wout Boerjan, John Ralph, Marie Baucher "Lignin Biosynthesis" Annu. Rev. Plant Biol. 2003, vol. 54, pp. 519–46. doi:10.1146/annurev.arplant.54.031902.134938
- ↑ 3.0 3.1 Li, Laigeng; Cheng, Xiao Fei; Leshkevich, Jacqueline; Umezawa, Toshiaki; Harding, Scott A.; Chiang, Vincent L. (2001). "The Last Step of Syringyl Monolignol Biosynthesis in Angiosperms is Regulated by a Novel Gene Encoding Sinapyl Alcohol Dehydrogenase". The Plant Cell 13 (7): 1567–1586. doi:10.1105/tpc.010111. PMID 11449052.
- ↑ Keeling, C. I.; Slessor, K. N.; Higo, H. A.; Winston, M. L. (2003). "Isolation and identification of new components of the honey bee (Apis mellifera L.) queen retinue pheromone". Proc. Natl. Acad. Sci. U.S.A. 100 (8): 4486–4491. doi:10.1073/pnas.0836984100. PMID 12676987. Bibcode: 2003PNAS..100.4486K.
- ↑ Davin, L. B. et al. (1997). "Stereoselective bimolecular phenoxy radical coupling by an auxiliary (dirigent) protein without an active center". Science 275 (5298): 362–366. doi:10.1126/science.275.5298.362. PMID 8994027.
- ↑ Pickel, B.; Constantin, M.-A.; Pfannsteil, J.; Conrad, J.; Beifuss, U.; Schaffer, A. (March 2007). "An Enantiocomplementary Dirigent Protein for the Enantioselective Laccase-Catalyzed Oxidative Coupling of Phenols". Angewandte Chemie 53 (4): 273–284. doi:10.1007/s10086-007-0892-x.
 |