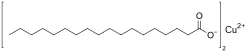
Chemistry:Copper(II) stearate
| |

| |
| Names | |
|---|---|
| Other names
copper(2+) dioctadecanoate, cupric stearate, copper distearate[1]
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Cu(C17H35COO)2 | |
| Molar mass | 630.48 |
| Appearance | blue-green amorphous substance |
| Density | 1.10 g/cm3 |
| Boiling point | 250 °C (482 °F; 523 K) |
| insoluble | |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P280, P305, P351, P338 | |
| Related compounds | |
Related compounds
|
Mercury(II) stearate, Cobalt(II) stearate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Copper(II) stearate is a metal-organic compound, a salt of copper and stearic acid with the formula Cu(C17H35COO)2.[2][3] The compound is classified as a metallic soap, i.e. a metal derivative of a fatty acid.
Synthesis
Exchange reaction of sodium stearate and copper sulfate:[4][5]
- [math]\displaystyle{ \mathsf{ CuSO_4 + 2C_{17}H_{35}O_2Na \ \xrightarrow{}\ Cu(C_{17}H_{35}O_2)_2\downarrow + Na_2SO_4 } }[/math]
Physical properties
Copper(II) stearate forms a blue-green amorphous substance[6] similar to plasticine both in appearance and touch.
Insoluble in water, ethanol, or ether; soluble in pyridine.[7]
Chemical properties
The compound is stable and non-reactive under normal conditions.[8]
When trying to ignite, copper stearate first melts and then begins to burn with a green (at the base) flame, then it quickly turns black due to the formation of cupric oxide:
- [math]\displaystyle{ \mathsf{ (C_{17}H_{35}COO)_2 Cu + 52O_2 \ \xrightarrow{t}\ CuO\downarrow + 36CO_2\uparrow + 35H_2O\uparrow } }[/math]
Uses
The compound is used in the production of antifouling paint and varnish materials.
Also used as a component in casting bronze sculptures.[9]
Also applies as a catalyst for the decomposition of hydroperoxides.[10]
References
- ↑ "CAS 660-60-6 Copper(ii)stearate - Alfa Chemistry". alfa-chemistry.com. https://www.alfa-chemistry.com/cas_660-60-6.htm.
- ↑ "Copper(II) stearate". Oakwood Chemical. https://www.oakwoodchemical.com/ProductsList.aspx?CategoryID=-2&txtSearch=150812&ExtHyperLink=1.
- ↑ "Copper(II) Stearate" (in en). American Elements. https://www.americanelements.com/copper-ii-stearate-660-60-6.
- ↑ Richardson, H. Wayne (16 January 1997) (in en). Handbook of Copper Compounds and Applications. CRC Press. p. 85. ISBN 978-0-8247-8998-5. https://books.google.com/books?id=Zk0z22smWUoC&dq=Copper(II)+stearate&pg=PA85. Retrieved 13 February 2023.
- ↑ "Cupric stearate | 660-60-6" (in en). ChemicalBook. https://www.chemicalbook.com/ChemicalProductProperty_EN_CB2258735.htm.
- ↑ "MatWeb - The Online Materials Information Resource". matweb.com. https://www.matweb.com/search/datasheet.aspx?matguid=6cc551c1b5e64216a059aa95f30af166&ckck=1.
- ↑ Haynes, William M. (9 June 2015) (in en). CRC Handbook of Chemistry and Physics, 96th Edition. CRC Press. p. 4-62. ISBN 978-1-4822-6097-7. https://books.google.com/books?id=RpLYCQAAQBAJ&dq=Copper(II)+stearate&pg=SA4-PA62. Retrieved 13 February 2023.
- ↑ "SAFETY DATA SHEET". chemservice.com. http://cdn.chemservice.com/product/msdsnew/External/English/NG-S99%20English%20SDS%20US.pdf.
- ↑ Scott, David A. (2002) (in en). Copper and Bronze in Art: Corrosion, Colorants, Conservation. Getty Publications. p. 293. ISBN 978-0-89236-638-5. https://books.google.com/books?id=yQKuSOzkLvcC&dq=Copper(II)+stearate&pg=PA293. Retrieved 13 February 2023.
- ↑ Ugo, R. (6 December 2012) (in en). Aspects of Homogeneous Catalysis: A Series of Advances. Springer Science & Business Media. p. 85. ISBN 978-94-010-1199-0. https://books.google.com/books?id=y_rtCAAAQBAJ&dq=Copper(II)+stearate+catalyst&pg=PA85. Retrieved 13 February 2023.
 |

