Chemistry:Copper(II) thiocyanate
 Copper(II) thiocyanate
| |
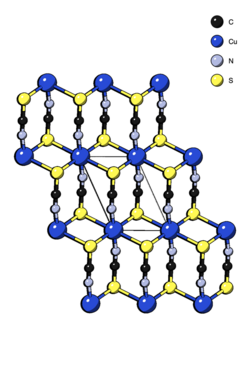 Crystal structure of copper(II) thiocyanate
| |
| Names | |
|---|---|
| Other names
Cupric thiocyanate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| Cu(SCN)2 | |
| Molar mass | 179.71 g/mol[1] |
| Appearance | black powder |
| Density | 2.47 g/cm3[1] |
| Melting point | decomposes at 180 C[2] |
| Insoluble | |
| 0.66×10−3 cm3/mol[1] | |
| Related compounds | |
Other anions
|
Copper(II) bromide, Copper(II) chloride |
Other cations
|
Copper(I) thiocyanate, Cobalt(II) thiocyanate, Mercury(II) thiocyanate, Ammonium thiocyanate Potassium thiocyanate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Copper(II) thiocyanate (or cupric thiocyanate) is a coordination polymer with formula Cu(SCN)2.[1] It is a black solid which slowly decomposes in moist air.[2] It was first reported in 1838 by Karl Ernst Claus and its structure was determined first in 2018.[3][1]
Structure
The structure of Cu(SCN)2 was determined via powder X-ray diffraction and consists of chains of Cu(NCS)2 linked together by weak Cu–S–Cu bonds into two-dimensional layers. It can be considered a Jahn–Teller distorted analogue of the mercury thiocyanate structure-type. Each copper is octahedrally coordinated by four sulfurs and two nitrogens. The sulfur end of the SCN− ligand is doubly bridging.[1]
Synthesis
Copper(II) thiocyanate can be prepared from the reaction of concentrated solutions of copper(II) and a soluble thiocyanate salt in water, precipitating as a black powder.[2][3] With rapid drying, pure Cu(SCN)2 can be isolated. Reaction at lower concentrations and for longer periods of time generates instead copper(I) thiocyanate.[4]
Magnetism
Copper(II) thiocyanate, like copper(II) bromide and copper(II) chloride, is a quasi low-dimensional antiferromagnet and it orders at 12 K (−261 °C) into a conventional Néel ground state.[1]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Cliffe, Matthew J.; Lee, Jeongjae; Paddison, Joseph A. M.; Schott, Sam; Mukherjee, Paromita; Gaultois, Michael W.; Manuel, Pascal; Sirringhaus, Henning et al. (2018-04-25). "Low-dimensional quantum magnetism in Cu(NCS)2: A molecular framework material" (in en). Physical Review B 97 (14): 144421. doi:10.1103/PhysRevB.97.144421. ISSN 2469-9950.
- ↑ 2.0 2.1 2.2 Hunter, J. A.; Massie, W. H. S.; Meiklejohn, J.; Reid, J. (1969-01-01). "Thermal rearrangement in copper(II) thiocyanate". Inorganic and Nuclear Chemistry Letters 5 (1): 1–4. doi:10.1016/0020-1650(69)80226-6. ISSN 0020-1650. https://dx.doi.org/10.1016%2F0020-1650%2869%2980226-6.
- ↑ 3.0 3.1 Claus, C. (1838). "Beiträge zur näheren Kenntniss der Schwefelcyanmetalle" (in en). Journal für Praktische Chemie 15 (1): 401–411. doi:10.1002/prac.18380150142. ISSN 1521-3897. https://onlinelibrary.wiley.com/doi/abs/10.1002/prac.18380150142.
- ↑ Smith, D. L.; Saunders, V. I. (15 March 1982). "Preparation and structure refinement of the 2H polytype of β-copper(I) thiocyanate". Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry 38 (3): 907–909. doi:10.1107/S0567740882004361.
 |

