Chemistry:Cumene hydroperoxide
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Phenylpropane-2-peroxol | |
| Other names
Cumyl hydroperoxide
CHP | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C9H12O2 | |
| Molar mass | 152.193 g·mol−1 |
| Appearance | Colorless to pale yellow liquid |
| Density | 1.02 g/cm3 |
| Melting point | −9 °C (16 °F; 264 K) |
| Boiling point | 153 °C (307 °F; 426 K) |
| 1.5 g/100 mL | |
| Vapor pressure | 14 mmHg, at 20 °C |
| Hazards | |
| Safety data sheet | sigmaaldrich.com |
| GHS pictograms |     
|
| GHS Signal word | DANGER |
| H242, H302, H312, H314, H331, H373, H411 | |
| P220, P261, P273, P280, P305+351+338, P310 | |
| NFPA 704 (fire diamond) | |
| Flash point | 57 °C (135 °F; 330 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
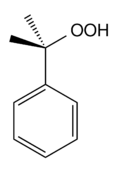
Cumene hydroperoxide is an organic hydroperoxide intermediate in the cumene process for synthesizing phenol and acetone from benzene and propene. It is typically used as an oxidizing agent.[2] Products of decomposition of cumene hydroperoxide are methylstyrene, acetophenone, and cumyl alcohol.[3] Its formula is C6H5C(CH3)2OOH.
One of the key uses for the material is as a free radical initiator for acrylate and methacrylate monomers, and polyester resins.
Cumene hydroperoxide is involved as an organic peroxide in the manufacturing of propylene oxide by the oxidation of propylene. This technology was commercialized by Sumitomo Chemical.[4] Oxidation of cumene affords cumene hydroperoxide
- C6H5(CH3)2CH + oxidation → C6H5(CH3)2COOH
The oxidation by cumene hydroperoxide of propylene affords propylene oxide and the byproduct cumyl alcohol. The reaction follows this stoichiometry:
- CH3CHCH2 + C6H5(CH3)2COOH → CH3CHCH2O + C6H5(CH3)2COH
Dehydrating and hydrogenating cumyl alcohol recycles the cumene.
Public safety
Cumene hydroperoxide[5] is believed to be one of the chemicals of concern[6] at the Arkema facility in Crosby, Texas in the aftermath of Hurricane Harvey.
References
- ↑ University, Safety Officer in Physical Chemistry at Oxford (2005). "Safety (MSDS) data for cumene hydroperoxide". http://msds.chem.ox.ac.uk/CU/cumene_hydroperoxide.html.
- ↑ Richard J. Lewis, Richard J. Lewis (Sr.), Hazardous chemicals desk reference, Publisher Wiley-Interscience, 2008, ISBN:0-470-18024-2, ISBN:978-0-470-18024-2, 1953 pages (page 799)
- ↑ Cumene Hydroperoxide at the Organic Chemistry Portal
- ↑ "Summary of Sumitomo process from Nexant Reports". http://nexant.ecnext.com/coms2/gi_0255-227/Developments-in-Propylene-Oxide-Technology.html.
- ↑ "The Rachel Maddow Show". MSNBC. 30 August 2017.
- ↑ Bagg, Julia; Johnson, Alex; Cumming, Jason (31 August 2017). "Crosby, Texas, Chemical Plant Explodes Twice, Arkema Group Says". NBC News. https://www.nbcnews.com/storyline/hurricane-harvey/harvey-danger-major-chemical-plant-near-houston-likely-explode-facility-n797581.
Related terms
- Cumene process
- 2-hydroperoxypropan-2-ylbenzene
External links
- Cumene hydroperoxide at International Chemical Safety Cards


