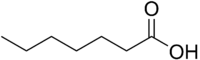
Chemistry:Enanthic acid
| |
| Names | |
|---|---|
| Preferred IUPAC name
Heptanoic acid | |
| Other names
Enthanoic acid; Enthanylic acid; Heptoic acid; Heptylic acid; Oenanthic acid; Oenanthylic acid; 1-Hexanecarboxylic acid; C7:0 (lipid numbers)
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C7H14O2 | |
| Molar mass | 130.187 g·mol−1 |
| Appearance | colorless oily liquid |
| Density | 0.9181 g/cm3 (20 °C) |
| Melting point | −7.5 °C (18.5 °F; 265.6 K) |
| Boiling point | 223 °C (433 °F; 496 K) |
| 0.2419 g/100 mL (15 °C) | |
| −88.60·10−6 cm3/mol | |
| Hazards | |
| GHS pictograms |  [2] [2]
|
| GHS Signal word | Danger[2] |
| H314[2] | |
| P261, P271, P280, P303+361+353, P304+340+310, P305+351+338[3] | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
6400 mg/kg (oral, rat) |
| Related compounds | |
Related compounds
|
Hexanoic acid, Octanoic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Enanthic acid, also called heptanoic acid, is an organic compound composed of a seven-carbon chain terminating in a carboxylic acid functional group. It is a colorless oily liquid with an unpleasant, rancid odor.[1] It contributes to the odor of some rancid oils. It is slightly soluble in water, but very soluble in ethanol and ether. Salts and esters of enanthic acid are called enanthates or heptanoates.
Its name derives from the Latin oenanthe which is in turn derived from the Ancient Greek oinos "wine" and anthos "blossom."
Production
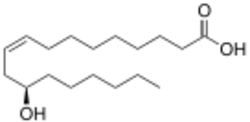
The methyl ester of ricinoleic acid, obtained from castor bean oil, is the main commercial precursor to enanthic acid. It is pyrolyzed to the methyl ester of 10-undecenoic acid and heptanal, which is then air-oxidized to the carboxylic acid. Approximately 20,000 tons were consumed in Europe and US in 1980.[4]
Laboratory preparations of enanthic acid include permanganate oxidation of heptanal[5] and 1-octene.[6]
Uses
Enanthic acid is used in the preparation of esters, such as ethyl enanthate, which are used as artificial flavors. Enanthic acid is used to esterify anabolic steroids in the preparation of drugs such as testosterone enanthate, trenbolone enanthate, drostanolone enanthate, and methenolone enanthate.
The triglyceride of enanthic acid is known as triheptanoin, which is a medical food.
Safety
Enanthic acid is corrosive.[2]
See also
References
- ↑ 1.0 1.1 Merck Index, 11th Edition, 4581
- ↑ 2.0 2.1 2.2 2.3 "Heptanoic Acid - Pubchem". https://pubchem.ncbi.nlm.nih.gov/compound/Heptanoic-acid.
- ↑ "Heptanoic acid". https://www.sigmaaldrich.com/product/aldrich/75190.
- ↑ David J. Anneken, Sabine Both, Ralf Christoph, Georg Fieg, Udo Steinberner, Alfred Westfechtel "Fatty Acids" in Ullmann's Encyclopedia of Industrial Chemistry 2006, Wiley-VCH, Weinheim. doi:10.1002/14356007.a10_245.pub2
- ↑ John R. Ruhoff (1936). "N-Heptanoic Acid". Organic Syntheses 16: 39. doi:10.15227/orgsyn.016.0039.
- ↑ Donald G. Lee, Shannon E. Lamb, Victor S. Chang (1981). "Carboxylic Acids from the Oxidation of Terminal Alkenes by Permanganate: Nonadecanoic Acid". Organic Syntheses 60: 11. doi:10.15227/orgsyn.060.0011.
 |

