Chemistry:Eosin Y
From HandWiki
Short description: Chemical compound
| |

| |
| Names | |
|---|---|
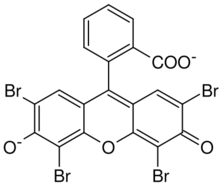
| IUPAC name
2-(2,4,5,7-tetrabromo-6-oxido-3-oxo-3H-xanthen-9-yl)benzoate [in its deprotonated form]
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| MeSH | Eosine+Yellowish-(YS) |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C20H6Br4Na2O5 | |
| Molar mass | 647.89052 |
| Appearance | Red powder |
| Density | 1.018 g·cm−3 |
| Melting point | 295.5 °C (563.9 °F; 568.6 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Eosin Y, also called C.I. 45380[1][2] or C.I. Acid Red 87,[2][1] is a member of the triarylmethane dyes. It is produced from fluorescein by bromination.[3]
Use
Eosin Y is commonly used as the red dye in red inks.
It is commonly used in histology, most notably in the H&E (Haematoxylin and Eosin) stain.[1] Eosin Y is also widely used in the Papanicolaou stain (or Pap stain used in the Pap test) and the Romanowsky type cytologic stains.[1][2] It is also used as a photosensitizer in organic synthesis.[4]
-
Eosin Y solution for staining microscopy slides.
-
Eosinophilic staining, using eosin Y, compared to other patterns when using hematoxylin and eosin (H&E).
See also
References
- ↑ 1.0 1.1 1.2 1.3 Bancroft, John; Stevens, Alan, eds (1982). The Theory and Practice of Histological Techniques (2nd ed.). Longman Group Limited.
- ↑ 2.0 2.1 2.2 Lillie, Ralph Dougall (1977). H. J. Conn's Biological stains (9th ed.). Baltimore: Williams & Wilkins. pp. 692p.
- ↑ Gessner, Thomas; Mayer, Udo (2000). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a27_179.
- ↑ Diercxsens, Nicolas (2017-04-10) (in en), Eosin Y, Chichester, UK: John Wiley & Sons, Ltd, pp. 1–3, doi:10.1002/047084289x.rn02033, ISBN 978-0-470-84289-8
 |



