Chemistry:Herrmann's catalyst
From HandWiki
Short description: Organopalladium compound used as a catalyst
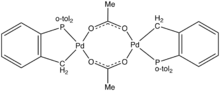
| |
| Identifiers | |
|---|---|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| Properties | |
| C46H46O4P2Pd2 | |
| Molar mass | 937.66 g·mol−1 |
| Appearance | yellow solid |
| Melting point | 123-125 °C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Herrmann's catalyst is an organopalladium compound that is a popular catalyst for the Heck reaction. It is a yellow air-stable solid that is soluble in organic solvents. Under conditions for catalysis, the acetate group is lost and the Pd-C bond undergoes protonolysis, giving rise to a source of "PdP(o-tol)
3".
The complex is made by reaction of tris(o-tolyl)phosphine with palladium(II) acetate:[1]
Many analogues of Hermann's catalyst have been developed, e.g. palladacycles obtained from 2-aminobiphenyl.[2]
References
- ↑ Herrmann, W. A.; Brossmer, C.; Reisinger, C.-P.; Riermeier, T. H.; Öfele, K.; Beller, M. (1997). "Palladacycles: Efficient New Catalysts for the Heck Vinylation of Aryl Halides". Chemistry – A European Journal 3: 1357–1364. doi:10.1002/chem.19970030823.
- ↑ Bruneau, A.; Roche, M.; Alami, M.; Messaoudi, S. (2015). "2-Aminobiphenyl Palladacycles: The "Most Powerful" Precatalysts in C–C and C–Heteroatom Cross-Couplings". ACS Catalysis 5: 1386–1396. doi:10.1021/cs502011x.
 |
