Chemistry:Iohexol
 | |
| Clinical data | |
|---|---|
| Trade names | Omnipaque, Hexopaque, Oraltag, others |
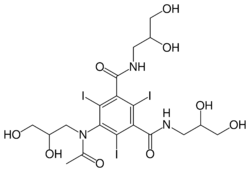
| Other names | 5-[N-(2,3-Dihydroxypropyl)acetamido]-2,4,6-triiodo-N,N'-bis(2,3-dihydroxypropyl)isophthalamide |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| License data | |
| Pregnancy category |
|
| Routes of administration | intrathecal, intravascular, by mouth, intracavital, rectal |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | Low |
| Metabolism | Nil |
| Elimination half-life | Variable |
| Excretion | Kidney, unchanged |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C19H26I3N3O9 |
| Molar mass | 821.142 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 174 to 180 °C (345 to 356 °F) |
| |
| |
| | |
Iohexol, sold under the trade name Omnipaque among others, is a contrast agent used for X-ray imaging.[1] This includes when visualizing arteries, veins, ventricles of the brain, the urinary system, and joints, as well as during computed tomography (CT scan).[1] It is given by mouth, injection into a vein, or into a body cavity.[2]
Side effects include vomiting, skin flushing, headache, itchiness, kidney problems, and low blood pressure.[1] Less commonly allergic reactions or seizures may occur.[1] Allergies to povidone-iodine or shellfish do not affect the risk of side effects more than other allergies.[3] Use in the later part of pregnancy may cause hypothyroidism in the baby.[4] Iohexol is an iodinated non-ionic radiocontrast agent.[1] It is in the low osmolar family.[5]
Iohexol was approved for medical use in 1985.[6] It is on the World Health Organization's List of Essential Medicines.[7][2]
Chemistry
The osmolality of iohexol ranges from 322 mOsm/kg—approximately 1.1 times that of blood plasma—to 844 mOsm/kg, almost three times that of blood.[8] Despite this difference, iohexol is still considered a low-osmolality contrast agent; the osmolality of older agents, such as diatrizoate, may be more than twice as high.[9]
Adverse effects
The most common side effects after intravenous injections are: pain at the site of injection (3%), blurring of vision (2%), nausea (2%), arrhythmia (2%), taste perversion (1%), hypotension (0.7%), and vomiting (0.7%).[10]
Society and culture
Naming
It is sold under the brand names Omnipaque.[11] It is also sold as a density gradient medium under the names Accudenz, Histodenz, and Nycodenz.[12][13]
Available forms
It is available in various concentrations, from 140[10] to 350[14] milligrams of iodine per milliliter.[10] Iohexol can given as intrathecal, intravascular, oral, rectal, intraarticular, or into the body cavity.[10]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 WHO Model Formulary 2008. World Health Organization. 2009. pp. 317–8. ISBN 9789241547659.
- ↑ 2.0 2.1 Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. 2015. p. 171. ISBN 9781284057560.
- ↑ ACR Manual on Contrast Media v10.3. 2017. American College of Radiology. 2017. p. 6. ISBN 9781559030120. https://www.acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf. Retrieved 1 January 2018.
- ↑ (in en) Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. Lippincott Williams & Wilkins. 2011. p. 761. ISBN 9781608317080. https://books.google.com/books?id=OIgTE4aynrMC&pg=PA761.
- ↑ (in en) A Short Textbook of Clinical Imaging. Springer Science & Business Media. 2012. p. 235. ISBN 9781447117551. https://books.google.com/books?id=muflBwAAQBAJ&pg=PA235.
- ↑ (in en) Clinical Nephrotoxins: Renal Injury from Drugs and Chemicals. Springer Science & Business Media. 2013. p. 325. ISBN 9789401590884. https://books.google.com/books?id=tkPwCAAAQBAJ&pg=PA325.
- ↑ World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. 2019. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ GE Healthcare (May 2006). "Omnipaque (Iohexol) injection. Product label". DailyMed. U.S. National Library of Medicine. http://dailymed.nlm.nih.gov/dailymed/fdaDrugXsl.cfm?id=3465&type=display.
- ↑ Amersham Health (April 2006). "Hypaque (Diatrizoate Meglumine and Diatrizoate Sodium) injection, solution. Product label". DailyMed. U.S. National Library of Medicine. http://dailymed.nlm.nih.gov/dailymed/fdaDrugXsl.cfm?id=997&type=display.
- ↑ 10.0 10.1 10.2 10.3 "Highlights of prescribing information for Omnipaque". US Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/018956s101lbl.pdf.
- ↑ "Omnipaque". Ireland: Health Products Regulatory Authority. January 2018. https://www.hpra.ie/img/uploaded/swedocuments/666c85d0-2222-42f9-873f-a269d151e198.pdf.
- ↑ "HistoDenz". Product information sheet. Sigma-Aldrich. https://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Sigma/Product_Information_Sheet/d2158pis.pdf.
- ↑ "Nycodenz®: A universal density gradient medium". Axis-Shield Density Gradient Media.. http://www.axis-shield-density-gradient-media.com/Leaflet%20Nycodenz.pdf.
- ↑ (in German) Austria-Codex. Vienna: Österreichischer Apothekerverlag. 2020. Omnipaque 350 mg J/ml Infusionsflasche.
External links
- "Iohexol". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/iohexol.
- "Iohexol Injection, Oral, Rectal Advanced Patient Information". 13 January 2019. https://www.drugs.com/cons/iohexol-injection-oral-rectal.html.
 |
