Chemistry:Iron(II) acetate
| |
| Names | |
|---|---|
| IUPAC name
Iron(II) acetate
| |
| Other names
Ferrous acetate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| C4H6FeO4 | |
| Molar mass | 173.933 g·mol−1 |
| Appearance | White crystals (anhydrous) Light green crystals (tetrahydrate) |
| Odor | Odorless |
| Density | 1.734 g/cm3 (−73 °C)[1] |
| Melting point | 190–200 °C (374–392 °F; 463–473 K) decomposes[2][3] |
| Soluble[2] | |
| Structure | |
| Orthorhombic, oP75 (200 K) | |
| Pbcn, No. 60 (200 K)[1] | |
| 2/m 2/m 2/m (200 K) | |
a = 18.1715(4) Å, b = 22.1453(5) Å, c = 8.2781(2) Å (200 K) α = 90°, β = 90°, γ = 90°
| |
| Hazards | |
| GHS pictograms |  [3] [3]
|
| GHS Signal word | Warning |
| H315, H319, H335[3] | |
| P261, P305+351+338[3] | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Iron(II) acetate is a coordination complex with formula Fe(CH3COO)2. It is a white solid, although impure samples can be slightly colored.[1] A light green tetrahydrate is also known, which is highly soluble in water.
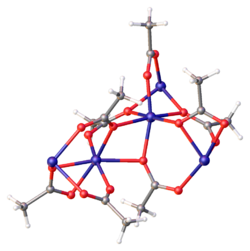
Preparation and structure
Iron powder reacts with acetic acid in electrolysis to give the ferrous acetate, with evolution of hydrogen gas:[1]
- Fe + 2 CH3CO2H → Fe(CH3CO2)2 + H2
It can also be made from the insoluble, olive green, Iron(II) carbonate.[citation needed]
It adopts a polymeric structure with octahedral Fe(II) centers interconnected by acetate ligands. It is a coordination polymer.[1]
A hydrated form be made by the reaction of ferrous oxide or ferrous hydroxide with acetic acid.[5]
Reaction of scrap iron with acetic acid affords a brown mixture of various iron(II) and iron(III) acetates that are used in dyeing.[6]
Uses
Ferrous acetate is used as a mordant by the dye industry. Ebonizing wood is one such process.[7]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Weber, Birgit; Betz, Richard; Bauer, Wolfgang; Schlamp, Stephan (2011). "Crystal Structure of Iron(II) Acetate". Zeitschrift für anorganische und allgemeine Chemie 637: 102–107. doi:10.1002/zaac.201000274.
- ↑ 2.0 2.1 Lide, David R., ed (2009). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. ISBN 978-1-4200-9084-0.
- ↑ 3.0 3.1 3.2 3.3 Sigma-Aldrich Co., Iron(II) acetate. Retrieved on 2014-05-03.
- ↑ "MSDS of Ferrous acetate". Fisher Scientific. https://www.fishersci.ca/viewmsds.do?catNo=AC305370500.
- ↑ "Synthesis of Iron(II) acetate hydrate (ferrous acetate)". http://www.ims.demokritos.gr/people/tbou/iron_acetate.html.
- ↑ Wildermuth, Egon; Stark, Hans; Friedrich, Gabriele; Ebenhöch, Franz Ludwig; Kühborth, Brigitte; Silver, Jack; Rituper, Rafael (2000). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a14_591.
- ↑ Ebonizing Wood with Ferric Acetate
Acetyl halides and salts of the acetate ion
| |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AcOH | He | ||||||||||||||||||
| LiOAc | Be(OAc)2 BeAcOH |
B(OAc)3 | AcOAc ROAc |
NH4OAc | AcOOH | FAc | Ne | ||||||||||||
| NaOAc | Mg(OAc)2 | Al(OAc)3 ALSOL Al(OAc)2OH Al2SO4(OAc)4 |
Si | P | S | ClAc | Ar | ||||||||||||
| KOAc | Ca(OAc)2 | Sc(OAc)3 | Ti(OAc)4 | VO(OAc)3 | Cr(OAc)2 Cr(OAc)3 |
Mn(OAc)2 Mn(OAc)3 |
Fe(OAc)2 Fe(OAc)3 |
Co(OAc)2, Co(OAc)3 |
Ni(OAc)2 | Cu(OAc)2 | Zn(OAc)2 | Ga(OAc)3 | Ge | As(OAc)3 | Se | BrAc | Kr | ||
| RbOAc | Sr(OAc)2 | Y(OAc)3 | Zr(OAc)4 | Nb | Mo(OAc)2 | Tc | Ru(OAc)2 Ru(OAc)3 Ru(OAc)4 |
Rh2(OAc)4 | Pd(OAc)2 | AgOAc | Cd(OAc)2 | In | Sn(OAc)2 Sn(OAc)4 |
Sb(OAc)3 | Te | IAc | Xe | ||
| CsOAc | Ba(OAc)2 | Hf | Ta | W | Re | Os | Ir | Pt(OAc)2 | Au | Hg2(OAc)2, Hg(OAc)2 |
TlOAc Tl(OAc)3 |
Pb(OAc)2 Pb(OAc)4 |
Bi(OAc)3 | Po | At | Rn | |||
| Fr | Ra | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |||
| ↓ | |||||||||||||||||||
| La(OAc)3 | Ce(OAc)x | Pr | Nd | Pm | Sm(OAc)3 | Eu(OAc)3 | Gd(OAc)3 | Tb | Dy(OAc)3 | Ho(OAc)3 | Er | Tm | Yb(OAc)3 | Lu(OAc)3 | |||||
| Ac | Th | Pa | UO2(OAc)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||||
 |

