Chemistry:Tris(acetylacetonato)iron(III)
| |
| |

| |
| Names | |
|---|---|
| IUPAC name
Tris(2,4-dioxopentan-3-ido-κ2O,O′)iron
| |
| Other names
Iron(III) acetylacetonate, Iron(III) tris(2,4-pentanedionato), Fe(acac)3
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| Fe(C5H7O2)3 | |
| Molar mass | 353.17 g/mol |
| Appearance | Red Solid |
| Density | 1.348 g/cm3 |
| Melting point | 180 to 181 °C (356 to 358 °F; 453 to 454 K) |
| Boiling point | decomposes |
| 2 g/L | |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| HH302 + H312 + H332Script error: No such module "Preview warning".Category:GHS errors, HH318Script error: No such module "Preview warning".Category:GHS errors | |
| PP261Script error: No such module "Preview warning".Category:GHS errors, PP280Script error: No such module "Preview warning".Category:GHS errors, PP301 + P312Script error: No such module "Preview warning".Category:GHS errors, PP302 + P352 + P312Script error: No such module "Preview warning".Category:GHS errors, PP304 + P340 + P312Script error: No such module "Preview warning".Category:GHS errors, PP305 + P351 + P338Script error: No such module "Preview warning".Category:GHS errors | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
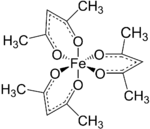
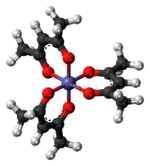
Tris(acetylacetonato) iron(III), often abbreviated Fe(acac)3, is a ferric coordination complex featuring acetylacetonate (acac) ligands, making it one of a family of metal acetylacetonates. It is a red air-stable solid that dissolves in nonpolar organic solvents.
Preparation
Fe(acac)3 is prepared by treating freshly precipitated Fe(OH)3 with acetylacetone.[2]
- Fe(OH)3 + 3 HC5H7O2 → Fe(C5H7O2)3 + 3 H2O
Structure and properties
Fe(acac)3 is an octahedral complex with six equivalent Fe-O bonds with bond distances of about 2.00 Å. The regular geometry is consistent with a high-spin Fe3+ core. As the metal orbitals are all evenly occupied the complex is not subject to Jahn-Teller distortions and thus adopts a D3 molecular symmetry. In contrast, the related metal acetylacetonate Mn(acac)3 adopts a more distorted octahedral structure.[3] The 5 unpaired d-electrons also result in the complex being paramagnetic, with a magnetic moment of 5.90 μB.
Fe(acac)3 possesses helical chirality. The Δ- and Λ-enantiomers slowly inter-convert via Bailar and Ray–Dutt twists. The rate of interconversion is sufficiently slow to allow its enantiomers to be partially resolved.[4]
Reactions
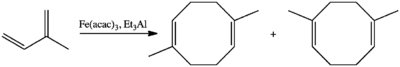
Fe(acac)3 has been examined as a precatalyst and reagent in organic chemistry, although the active iron-containing species is usually unidentified in these processes. In one instance, Fe(acac)3 was shown to promote cross-coupling a diene to an olefin.[5] Fe(acac)3 catalyzes the dimerization of isoprene to a mixture of 1,5-dimethyl-1,5-cyclooctadiene and 2,5-dimethyl-1,5-cyclooctadiene.[6]
Fe(acac)3 also catalyzes the ring-opening polymerization of 1,3-benzoxazine.[7] Beyond the area of polymerization, Fe(acac)3 has been found to catalyze the reaction of N-sulfonyl oxaziridines with olefins to form 1,3-oxazolidine products.[8]
References
- ↑ GHS: Sigma-Aldrich 517003
- ↑ US patent 2004127690, Chaudhari, Mihir Kanti et al., "Process for making metal acetylacetonates", issued 2004-07-01
- ↑ Lawson, K.E. (1961). "The infrared absorption spectra of metal acetylacetonates.". Spectrochimica Acta 17 (3): 248–258. doi:10.1016/0371-1951(61)80071-4. Bibcode: 1961AcSpe..17..248L.
- ↑ Anders Lennartson "Optical resolution and racemisation of [Fe(acac)3]" Inorganica Chimica Acta 2011, vol. 365, pp. 451–453. doi:10.1016/j.ica.2010.07.066
- ↑ Takacs, J. A., L.; Madhavan, G.V.; Creswell, M.; Seely, F.; Devroy, W. (1986). "Iron-Catalyzed Aminohydroxylation of Olefins". Organometallics 5 (11): 2395–2398. doi:10.1021/om00142a044.
- ↑ Misono, A. (1966). "Oligomerization of isoprene by cobalt or iron complex catalysts". Bulletin of the Chemical Society of Japan 39 (11): 2425–2429. doi:10.1246/bcsj.39.2425.
- ↑ Sudo, A.; Hirayama, Shoji; Endo, Takeshi (2010). "Highly efficient catalysts-acetylacetonato complexes of transition metals in the 4th period for ring-opening polymerization of 1,3-benzoxazine". Journal of Polymer Science Part A: Polymer Chemistry 48 (2): 479. doi:10.1002/pola.23810. Bibcode: 2010JPoSA..48..479S.
- ↑ Williamson, K. T.; Yoon, T. (2010). "Iron-Catalyzed Aminohydroxylation of Olefins". J. Am. Chem. Soc. 132 (13): 4570–4571. doi:10.1021/ja1013536. PMID 20232850.
 |