Chemistry:Ferric oxalate
| |
| Names | |
|---|---|
| Systematic IUPAC name
iron(3+) ethanedioate (2:3) | |
| Other names
Iron(III) oxalate
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6Fe2O12 | |
| Molar mass | 375.747 g/mol |
| Appearance | Pale yellow solid (anhydrous) Lime green solid (hexahydrate) |
| Odor | odorless |
| Melting point | 365.1 °C (689.2 °F) |
| slightly soluble | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
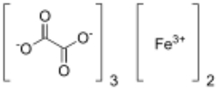
Ferric oxalate, also known as iron(III) oxalate, refers to inorganic compounds with the formula Fe
2(C
2O
4)
3(H2O)x but could also refer to salts of Fe(C
2O
4)
3]3-. Fe
2(C
2O
4)
3(H2O)x are coordination polymers with varying degrees of hydration. The coordination complex with the formula Fe(C
2O
4)
3]3- forms a variety of salts, a well-known example being potassium ferrioxalate. This article emphasizes the coordination polymers.
Structure
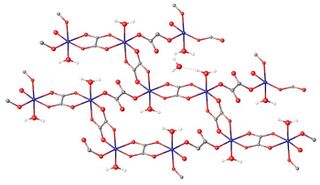
Tetrahydrate
According to X-ray crystallography of the tetrahydrate Fe
2(C
2O
4)
3 · 4 H
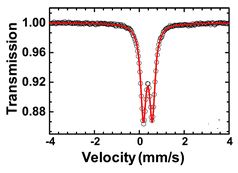
2O, iron is octahedral. The oxalate ligand]]s are bridging. Some through all four oxygen atoms, some with two oxygen atoms. Half of the water is lattice water, being situated between chains of Fe oxalates. Mössbauer spectrum of Fe
2(C
2O
4)
3 · 4 H
2O exhibits an isomer shift of 0.38 mm/s and a quadrupole splitting of 0.40 mm/s, suggesting a high spin Fe3+ in octahedral coordination.[1][2]
Production
Ferric oxalate may be produced by reaction of Iron (iii) hydroxide and solution of oxalic acid
2 Fe(OH)3 + 3 H2C2O4 = Fe2(C2O4)3 + 6 H2O
Uses
Dentistry
Like many oxalates, ferric oxalate has been investigated as a short-term treatment for dentin hypersensitivity.[3] It is used in certain toothpaste formulations; however, its effectiveness has been questioned.[4]
Photography
Ferric oxalate is used as the light-sensitive element in the Kallitype photographic printing process; and the platinotype process Platinum/Palladium Printing.
Batteries
Ferric oxalate tetrahydrate has been investigated as a possible cheap material for the positive electrode of lithium-iron batteries. It can intercalate lithium ions at an average potential of 3.35 V, and has shown a sustainable capacity of 98 mAh/g.[1]
Organic synthesis
Ferric oxalate hexahydrate is used with sodium borohydride for radical Markovnikov hydrofunctionalization reactions of alkenes.[5]
See also
A number of other iron oxalates are known:-
References
- ↑ 1.0 1.1 Ahouari, Hania; Rousse, Gwenaëlle; Rodríguez-Carvajal, Juan; Sougrati, Moulay-Tahar; Saubanère, Matthieu; Courty, Matthieu; Recham, Nadir; Tarascon, Jean-Marie (2015). "Unraveling the Structure of Iron(III) Oxalate Tetrahydrate and Its Reversible Li Insertion Capability". Chemistry of Materials 27 (5): 1631–1639. doi:10.1021/cm5043149.
- ↑ Rousse, G.; Rodríguez-Carvajal, J. (2016). "Oxalate-mediated long-range antiferromagnetism order in Fe2(C2O4)3·4H2O". Dalton Transactions 45 (36): 14311–14319. doi:10.1039/C6DT02740G. PMID 27539964.
- ↑ Gillam, D. G.; Newman, H. N.; Davies, E. H.; Bulman, J. S.; Troullos, E. S.; Curro, F. A. (2004). "Clinical evaluation of ferric oxalate in relieving dentine hypersensitivity". Journal of Oral Rehabilitation 31 (3): 245–250. doi:10.1046/j.0305-182X.2003.01230.x. PMID 15025657.
- ↑ Cunha-Cruz, J.; Stout, J. R.; Heaton, L. J.; Wataha, J. C. (29 December 2010). "Dentin Hypersensitivity and Oxalates: a Systematic Review". Journal of Dental Research 90 (3): 304–310. doi:10.1177/0022034510389179. PMID 21191127.
- ↑ Barker, Timothy (2001-04-15). "Ferric Oxalate Hexahydrate" (in en). Encyclopedia of Reagents for Organic Synthesis (1 ed.). Wiley. pp. 1–4. doi:10.1002/047084289X.rn02346. ISBN 978-0-471-93623-7. https://onlinelibrary.wiley.com/doi/book/10.1002/047084289X.
 |