Chemistry:Kojic acid
| |
| Names | |
|---|---|
| Preferred IUPAC name
5-Hydroxy-2-(hydroxymethyl)-4H-pyran-4-one | |
| Other names
Kojic acid, 5-Hydroxy-2-(hydroxymethyl)-4-pyrone, 2-hydroxymethyl-5-hydroxy-γ-pyrone
| |
| Identifiers | |
3D model (JSmol)
|
|
| 120895 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| EC Number |
|
| 3620 | |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| C6H6O4 | |
| Molar mass | 142.110 g·mol−1 |
| Appearance | white |
| Melting point | 152 to 155 °C (306 to 311 °F; 425 to 428 K) |
| Slight | |
| Acidity (pKa) | 9.40[1] |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H351 | |
| P201, P280, P308+313 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
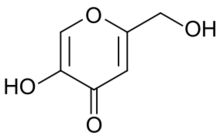
Kojic acid is an organic compound with the formula HOCH
2C
5H
2O
2OH. It is a derivative of 4-pyrone that functions in nature as a chelation agent produced by several species of fungi, especially Aspergillus oryzae, which has the Japanese common name koji.[2][3][4] Kojic acid is a by-product in the fermentation process of malting rice, for use in the manufacturing of sake, the Japanese rice wine.[2] It is a mild inhibitor of the formation of pigment in plant and animal tissues, and is used in food and cosmetics to preserve or change colors of substances.[5] It forms a bright red complex with ferric ions.[6]
Biosynthesis
13C-Labeling studies have revealed at least two pathways to kojic acid. In the usual route, dehydratase enzymes convert glucose to kojic acid. Pentoses are also viable precursors in which case dihydroxyacetone is invoked as an intermediate.[2]
Applications
Kojic acid may be used on cut fruits to prevent oxidative browning, in seafood to preserve pink and red colors, and in cosmetics to lighten skin. As an example of the latter, it is used to treat skin diseases like melasma.[7] Kojic acid also has antibacterial and antifungal properties.[citation needed] The cocrystals of kojic acid with quercetin were found to have two times better cytotoxic activity to human cervical cancer cells (HeLa) and human colon cancer cells (Caco-2) in comparison with quercetin itself.[8]
Other effects
Kojic acid has been shown to protect Chinese hamster ovary cells against ionizing radiation-induced damage. When exposed to a lethal dose of 3 Gy gamma radiation, dogs pretreated with kojic acid had a 51-day survival rate of 66.7% while the control group died within 16 days.[9]
Chemical reactions

Deprotonation of the ring-OH group converts kojic acid to kojate. Kojate chelates to iron(III), forming a red complex Fe(HOCH
2C
5OH
2O
2)
3. This kind of reaction may be the basis of the biological function of kojic aicd, that is, to solubilize ferric iron.[10]
Being a multifunctional molecule, kojic acid has diverse organic chemistry. The hydroxymethyl group gives the chloromethyl derivative upon treatment with thionyl chloride.[11]
References
- ↑ Bjerrum, J., et al. Stability Constants, Chemical Society, London, 1958.
- ↑ 2.0 2.1 2.2 Bentley, R. (2006). "From miso, sake and shoyu to cosmetics: a century of science for kojic acid". Nat. Prod. Rep. 23 (6): 1046–1062. doi:10.1039/b603758p. PMID 17119644.
- ↑ Yabuta T (1924). "The constitution of kojic acid, a γ-pyrone derivative formed by Aspergillus oryzae from carbohydrates". Journal of the Chemical Society 125: 575–587. doi:10.1039/ct9242500575.
- ↑ Parvez, Shoukat; Kang, Moonkyu; Chung, Hwan-Suck; Cho, Chongwoon; Hong, Moo-Chang; Shin, Min-Kyu; Bae, Hyunsu (2006). "Survey and mechanism of skin depigmenting and lightening agents". Phytotherapy Research 20 (11): 921–34. doi:10.1002/ptr.1954. PMID 16841367.
- ↑ "Kojic acid and enzymatic browning"]. Food and Agriculture Organization of the United Nations. 2000. http://www.fao.org/ag/Ags/agsi/ENZYMEFINAL/Enzymatic%20Browning.html#KOJIC.
- ↑ Nurchi, Valeria M.; Lachowicz, Joanna I.; Crisponi, Guido; Murgia, Sergio; Arca, Massimiliano; Pintus, Anna; Gans, Peter; Niclos-Gutierrez, Juan et al. (2011-05-27). "Kojic acid derivatives as powerful chelators for iron(III) and aluminium(III)" (in en). Dalton Transactions 40 (22): 5984–5998. doi:10.1039/C1DT00005E. ISSN 1477-9234. PMID 21552634.
- ↑ Melasma , American Academy of Dermatology
- ↑ Veverka, M., Dubaj, T., Gallovič, J., Jorík, V., Veverková, E., Danihelová, M., & Šimon, P. (2015). Cocrystals of quercetin: synthesis, characterization, and screening of biological activity. Monatshefte für Chemie-Chemical Monthly,146(1), 99-109 doi:10.1007/s00706-014-1314-6
- ↑ Wang, Kai; Li, Peng-Fei; Han, Chun-Guang; Du, Li; Liu, Chao; Hu, Ming; Lian, Shi-Jie; Liu, Yong-Xue (2014). "Protective Effects of Kojic Acid on the Periphery Blood and Survival of Beagle Dogs after Exposure to a Lethal Dose of Gamma Radiation". Radiation Research 182 (6): 666–673. doi:10.1667/RR13823.1. PMID 25409121. Bibcode: 2014RadR..182..666W.
- ↑ Zaremba, K.; Lasocha, W.; Adamski, A.; Stanek, J.; Pattek-Janczyk, A. (2007). "Crystal Structure and Magnetic Properties of Tris(2-hydroxymethyl-4-oxo-4H-pyran- 5-olato-κ2O5,O4)iron(III)". Journal of Coordination Chemistry 60 (14): 1537–1546. doi:10.1080/00958970601084243.
- ↑ Agyemang, Nana; Murelli, Ryan P. (2019). "Synthesis of 5-Hydroxy-4-methoxy-2-methylpyrylium Trifluoromethanesulfonate from Kojic Acid". Organic Syntheses 96: 494–510. doi:10.15227/orgsyn.096.0494.
External links
- Safety MSDS data
- Mohajer, Fatemeh; Mohammadi Ziarani, Ghodsi (2021). "An Overview of Quantitative and Qualitative Approaches on the Synthesis of Heterocyclic Kojic Acid Scaffolds through the Multi-Component Reactions". Heterocycles (Japan Institute of Heterocyclic Chemistry) 102 (2): 211. doi:10.3987/REV-20-936. https://www.heterocycles.jp/newlibrary/downloads/PDF/26925/102/2.
 |

