Chemistry:Meso-Octamethylporphyrinogen
| |
| Names | |
|---|---|
| IUPAC name
5,5,10,10,15,15,20,20-octamethyl-21,22,23,24-tetrahydroporphyrin
| |
| Other names
acetonepyrrole, octamethylcalix[4]pyrrole
| |
| Identifiers | |
3D model (JSmol)
|
|
| 365398 | |
| ChEBI | |
| ChemSpider | |
| EC Number |
|
| 2142403 | |
PubChem CID
|
|
| |
| |
| Properties | |
| C28H36N4 | |
| Molar mass | 428.624 g·mol−1 |
| Appearance | white solid |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
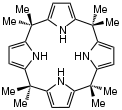
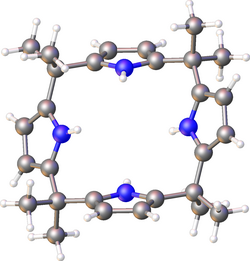
meso-Octamethylporphyrinogen, usually referred to simply as octamethylporphyrinogen, is an organic compound with the formula (Me2C-C4H2NH)4 (Me = CH3. It is one of the simplest porphyrinogens, a family of compounds that occur as intermediates in the biosynthesis of hemes and chlorophylls. In contrast to those rings, porphyrinogens are colorless since they lack extended conjugation. The prefix meso-octamethyl indicates that the eight methyl groups are located on the carbon centers that interconnect the four pyrrole rings. Also unlike porphyrins, the porphyrinogens are highly ruffled.[1]
Preparation
The compound was first reported by Adolph Bayer.[2] It is made by condensation of pyrrole with acetone.[3]
Reactions
The pyrrolic N-H centers of ctamethylporphyrinogen can be deprotonated, and the resulting tetraanion functions as a tetradentate ligand for a variety of metal ions.[5]
References
- ↑ Gale, Philip A.; Sessler, Jonathan L.; Král, Vladimír (1998). "Calixpyrroles". Chemical Communications: 1–8. doi:10.1039/A706280J.
- ↑ Baeyer, Adolf (1886). "Ueber ein Condensationsproduct von Pyrrol mit Aceton". Berichte der Deutschen Chemischen Gesellschaft 19 (2): 2184–2185. doi:10.1002/cber.188601902121. https://zenodo.org/record/2220337.
- ↑ Sobral, Abilio J.F.N. (2005). "Synthesis of Meso-Octamethylporphyrinogen: An Undergraduate Laboratory Mini-Scale Experiment in Organic Heterocyclic Chemistry". Journal of Chemical Education 82 (4): 618. doi:10.1021/ed082p618. Bibcode: 2005JChEd..82..618S.
- ↑ Allen, William E.; Gale, Philip A.; Brown, Christopher T.; Lynch, Vincent M.; Sessler, Jonathan L. (1996). "Binding of Neutral Substrates by Calix[4]pyrroles". Journal of the American Chemical Society 118 (49): 12471–12472. doi:10.1021/ja9632217.
- ↑ Bachmann, Julien; Hodgkiss, Justin M.; Young, Elizabeth R.; Nocera, Daniel G. (2007). "Ground- and Excited-State Reactivity of Iron Porphyrinogens". Inorganic Chemistry 46 (3): 607–609. doi:10.1021/ic0616636. PMID 17256999.
 |

