Chemistry:Molybdenum(III) bromide
From HandWiki
| |
| |
| Names | |
|---|---|
| IUPAC name
Molybdenum(III) bromide
| |
| Other names
Molybdenum tribromide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| MoBr3 | |
| Molar mass | 335.70 g/mol |
| Appearance | dark green to black solid |
| Density | 4.89 g/cm3 |
| Melting point | 500 °C (932 °F; 773 K) (decomposes) |
| insoluble | |
| Solubility | soluble in pyridine |
| +525.0·10−6 cm3/mol | |
| Related compounds | |
Other anions
|
Molybdenum(III) chloride Molybdenum(III) iodide |
Related compounds
|
Molybdenum(II) bromide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Molybdenum(III) bromide is the inorganic compound with the formula MoBr3. It is a black solid that is insoluble in most solvents but dissolves in donor solvents such as pyridine.
Preparation
Molybdenum(III) bromide is produced by the reaction of elemental molybdenum and bromine at 350 °C (662 °F).[1]
It can also be prepared from the reduction of molybdenum(IV) bromide with molybdenum metal, hydrogen gas, or a hydrocarbon.[2]
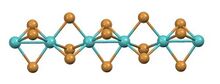
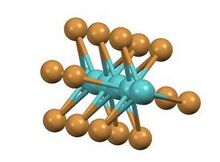
It has a structure consisting of infinite chains of face-sharing octahedra with alternatingly short and long Mo-Mo contacts. The same structure is adopted by the tribromides of ruthenium and technetium.[3][4] In contrast, in the high temperature phase of titanium(III) iodide, the Ti---Ti separation is invariant.
References
- ↑ F. Hein, S. Herzog "Molybdenum(III) Bromide" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 1407.
- ↑ Perry, Dale L. (2011). Handbook of Inorganic Compounds (2nd ed.). Boca Raton: Taylor & Francis. pp. 279. ISBN 978-1-4398-1461-1.
- ↑ Dietrich Babel: Die Verfeinerung der MoBr3-Struktur (Refinement of the MoBr3-Structure) In: Journal of Solid State Chemistry. 1972, volume 4, S. 410–416, doi:10.1016/0022-4596(72)90156-9.
- ↑ Order-Disorder Transformation in RuBr3 and MoBr3: A two-Dimensional Ising Model" Merlino, S.; Labella, L.; Marchetti, F.; Toscani, S. Chemistry of Materials 2004, volume 16, p3895-p3903
 |

