Chemistry:Molybdenum(III) iodide
| |
| Names | |
|---|---|
| IUPAC names
Molybdenum(III) iodide
Molybdenum triiodide | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| MoI3 | |
| Molar mass | 476.65 g/mol |
| Appearance | black solid[1] |
| Melting point | 927 °C (1,701 °F; 1,200 K) [1] (decomposes) |
| insoluble | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Molybdenum(III) iodide is the inorganic compound with the formula MoI3.
Preparation
Molybdenum(III) iodide is created by the reaction of molybdenum hexacarbonyl with iodine gas at 105 °C (221 °F).[2]
- 2 Mo(CO)6 + 3 I2 → 2 MoI3 + 12 CO
It can also be made from molybdenum(V) chloride and a solution of hydrogen iodide in carbon disulfide.
- MoCl5 + 5 HI → MoI3 + 5 HCl + I2
A further method is direct reaction between molybdenum metal and excess iodine at 300 °C (572 °F).
- 2 Mo + 3 I2 → 2 MoI3
As molybdenum(III) iodide is the highest stable iodide of molybdenum, this is the preferred route.[1]
Properties
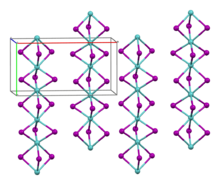
Molybdenum(III) iodide is a black antiferromagnetic solid that is air-stable at room temperature. In vacuum, it decomposes above 100 °C to molybdenum(II) iodide and iodine. It is insoluble in polar and non-polar solvents.[2] Its crystal structure is isotypic with zirconium(III) iodide.[3]
References
- ↑ 1.0 1.1 1.2 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 1019-1021. ISBN 978-0-08-037941-8.
- ↑ 2.0 2.1 hrsg. von Georg Brauer. Unter Mitarb. von M. Baudler (1981). Handbuch der präparativen anorganischen Chemie / 3. (3rd ed.). Stuttgart: Enke. pp. 1539. ISBN 3-432-87823-0. OCLC 310719495. https://www.worldcat.org/oclc/310719495.
- ↑ Riedel, Erwin; Christoph, Janiak; Meyer, Hans-Jürgen (2012). Riedel moderne anorganische Chemie. Riedel, Erwin, 1930-, Janiak, Christoph., Meyer, Hans-Jürgen. (4. Aufl ed.). Berlin: De Gruyter. pp. 357. ISBN 978-3-11-024900-2. OCLC 781540844. https://www.worldcat.org/oclc/781540844.
| HI | He | ||||||||||||||||
| LiI | BeI2 | BI3 | CI4 | NI3 | I2O4, I2O5, I4O9 |
IF, IF3, IF5, IF7 |
Ne | ||||||||||
| NaI | MgI2 | AlI3 | SiI4 | PI3, P2I4 |
S | ICl, ICl3 |
Ar | ||||||||||
| KI | CaI2 | Sc | TiI4 | VI3 | CrI3 | MnI2 | FeI2 | CoI2 | NiI2 | CuI | ZnI2 | Ga2I6 | GeI2, GeI4 |
AsI3 | Se | IBr | Kr |
| RbI | SrI2 | YI3 | ZrI4 | NbI5 | Mo | Tc | Ru | Rh | Pd | AgI | CdI2 | InI3 | SnI4, SnI2 |
SbI3 | TeI4 | I | Xe |
| CsI | BaI2 | HfI4 | TaI5 | W | Re | Os | Ir | Pt | AuI | Hg2I2, HgI2 |
TlI | PbI2 | BiI3 | Po | AtI | Rn | |
| Fr | RaI2 | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La | Ce | Pr | Nd | Pm | SmI2 | Eu | Gd | TbI3 | Dy | Ho | Er | Tm | Yb | Lu | |||
| Ac | ThI4 | Pa | UI3, UI4 |
Np | Pu | Am | Cm | Bk | Cf | EsI3 | Fm | Md | No | Lr | |||
 |

