Chemistry:Molybdenum(III) chloride
| |||
| Names | |||
|---|---|---|---|
| IUPAC names
Molybdenum(III) chloride
Molybdenum trichloride | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| MoCl3 | |||
| Molar mass | 202.30 g/mol | ||
| Appearance | dark red solid paramagnetic | ||
| Density | 3.58 g/cm3 | ||
| Melting point | 410 °C (770 °F; 683 K) (decomposes) | ||
| insoluble | |||
| Solubility | insoluble in ethanol, diethyl ether | ||
| +43.0·10−6 cm3/mol | |||
| Hazards | |||
| Flash point | Non-flammable | ||
| Related compounds | |||
Other anions
|
Molybdenum(III) fluoride Molybdenum(III) bromide Molybdenum(III) iodide | ||
Other cations
|
Chromium(IV) chloride Tungsten(V) chloride | ||
Related molybdenum chlorides
|
Molybdenum(II) chloride Molybdenum(IV) chloride Molybdenum(V) chloride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Molybdenum(III) chloride is the inorganic compound with the formula MoCl3. It forms purple crystals.[1]
Synthesis and structure
Molybdenum(III) chloride is synthesized by the reduction of molybdenum(V) chloride with hydrogen.[2] A higher yield is produced by the reduction of pure molybdenum(V) chloride with anhydrous tin(II) chloride as the reducing agent.[3]
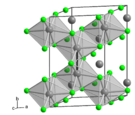
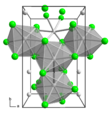
Molybdenum trichloride exists as two polymorphs: alpha (α) and beta (β). The alpha structure is similar to that of aluminum chloride (AlCl3). In this structure, molybdenum has octahedral coordination geometry and exhibits cubic close-packing in its crystalline structure. The beta structure, however, exhibits hexagonal close packing.[4]
Ether complexes
Molybdenum trichloride gives a ether complexes MoCl3(thf)3 and MoCl3(Et2O)3. They are beige, paramagnetic solids. Both feature octahedral Mo centers. The diethyl ether complex is synthesized by reducing a Et2O solution of MoCl5 with tin powder.[5] Older procedures involve stepwise reduction involving isolation of the Mo(IV)-thf complex.[6]
Hexa(tert-butoxy)dimolybdenum(III) is prepared by the salt metathesis reaction from MoCl3(thf)3:[7]
- 2 MoCl3(thf)3 + 6 LiOBu-t → Mo2(OBu-t)6 + 6 LiCl + 6 thf
References
- ↑ Perry, Dale L. (2011). Handbook of Inorganic Compounds (2nd ed.). Boca Raton: Taylor & Francis. pp. 279. ISBN 978-1-4398-1461-1.
- ↑ "Preparation of Trichloride and Tetrachloride of Molybdenum". Journal of Research of the National Bureau of Standards Section A 63A (2): 185–188. 1959. doi:10.6028/jres.063A.013. PMID 31216151.
- ↑ Larson, Melvin L. (1970). "Preparation of Some Metal Halides- Anhydrous Molybdenum Halides and Oxide Halides - A Summary". Inorganic Syntheses. 12. pp. 178–181.
- ↑ "Structural and scanning microscopy studies of layered compounds MCl3 (M= Mo, Ru, Cr) and MOCl2 (M= V, Nb, Mo, Ru, Os)". Journal of Alloys and Compounds 246 (1–2): 70–79. 1997. doi:10.1016/S0925-8388(96)02465-6.
- ↑ Maria, Sébastien; Poli, Rinaldo (2014). "Ether Complexes of Molybdenum(III) and Molybdenum(IV) chlorides". Inorganic Syntheses: Volume 36. Inorganic Syntheses. 36. pp. 15–18. doi:10.1002/9781118744994.ch03. ISBN 9781118744994. https://hal.archives-ouvertes.fr/hal-02042499/file/332-10.1002-9781118744994.ch03-Accepted.pdf.
- ↑ Dilworth, Jonathan R.; Richards, Raymond L. (1990). "The Synthesis of Molybdenum and Tungsten Dinitrogen Complexes". Inorganic Syntheses. Inorganic Syntheses. 28. pp. 33–43. doi:10.1002/9780470132593.ch7. ISBN 9780470132593.
- ↑ Broderick, Erin M.; Browne, Samuel C.; Johnson, Marc J. A. (2014). "Dimolybdenum and Ditungsten Hexa(Alkoxides)". Inorganic Syntheses: Volume 36. Inorganic Syntheses. 36. pp. 95–102. doi:10.1002/9781118744994.ch18. ISBN 9781118744994.
 |