Chemistry:Molybdenum(V) chloride
| |
| |
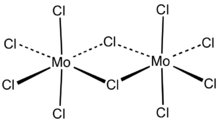
 Partially oxidized MoCl5
| |
| Names | |
|---|---|
| IUPAC names
Molybdenum(V) chloride
Molybdenum pentachloride | |
| Identifiers | |
3D model (JSmol)
|
|
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| Mo 2Cl 10 | |
| Molar mass | 273.21 g/mol (MoCl 5) |
| Appearance | dark-green solid hygroscopic paramagnetic |
| Density | 2.928 g/cm3 |
| Melting point | 194 °C (381 °F; 467 K) |
| Boiling point | 268 °C (514 °F; 541 K) |
| hydrolyzes | |
| Solubility | soluble in dry ether, dry alcohol, organic solvents |
| Structure | |
| monoclinic | |
| edge-shared bioctahedron | |
| Hazards | |
| Main hazards | oxidizer, hydrolyzes to release HCl |
| Flash point | Non-flammable |
| Related compounds | |
Other anions
|
|
Other cations
|
|
Related molybdenum chlorides
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Molybdenum(V) chloride is the inorganic compound with the empirical formula MoCl
5. This dark volatile solid is used in research to prepare other molybdenum compounds. It is moisture-sensitive and soluble in chlorinated solvents.
Structure
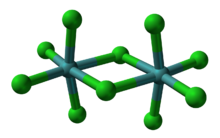
Usually called molybdenum pentachloride, it is in fact partly a dimer with the molecular formula Mo
2Cl
10.[1] In the dimer, each molybdenum has local octahedral symmetry and two chlorides bridge between the molybdenum centers.[2] A similar structure is also found for the pentachlorides of W, Nb and Ta.[3] In the gas phase and partly in solution, the dimers partially dissociate to give a monomeric MoCl
5.[4] The monomer is paramagnetic, with one unpaired electron per Mo center, reflecting the fact that the formal oxidation state is +5, leaving one valence electron on the metal center.
Preparation and properties
MoCl
5 is prepared by chlorination of Mo metal but also chlorination of MoO
3. The unstable hexachloride MoCl
6 is not produced in this way.[5]
MoCl
5 is reduced by acetonitrile to afford an orange acetonitrile complex, MoCl
4(CH
3CN)
2. This complex in turn reacts with THF to give MoCl
4(THF)
2, a precursor to other molybdenum-containing complexes.[6]
Molybdenum(IV) bromide is prepared by treatment of MoCl
5 with hydrogen bromide:
- 2 MoCl
5 + 10 HBr → 2 MoBr
4 + 10 HCl + Br
2
The reaction proceeds via the unstable molybdenum(V) bromide, which releases bromine at room temperature.[7]
MoCl
5 is a good Lewis acid toward non-oxidizable ligands. It forms an adduct with chloride to form [MoCl
6]−
. In organic synthesis, the compound finds occasional use in chlorinations, deoxygenation, and oxidative coupling reactions.[8]
Reactions
MoCl
5 is reduced by acetonitrile:[9]
- 2 MoCl
5 + 5 CH
3CN → 2 MoCl
4(CH
3CN)
2 + HCl + ClCH
2CN
Although it polymerizes tetrahydrofuran, MoCl
5 is stable in diethyl ether. Reduction of such solutions with tin gives MoCl
4((CH
3CH
2)
2O)
2 and MoCl
3((CH
3CH
2)
2O)
3, depending on conditions.[10]
Safety considerations
MoCl
5 is an aggressive oxidant and readily hydrolyzes to release HCl.
See also
- Molybdenum(IV) chloride
- Molybdenum(VI) chloride
- Molybdenum(V) fluoride
- Tungsten(V) chloride
References
- ↑ Holleman, A. F.; Wiberg, E. (2001). Inorganic Chemistry. San Diego, CA: Academic Press. ISBN 0-12-352651-5.
- ↑ Beck, J.; Wolf, F. (1997). "Three New Polymorphic Forms of Molybdenum Pentachloride". Acta Crystallogr. B53 (6): 895–903. doi:10.1107/S0108768197008331.
- ↑ Wells, A. E. (1984). Structural Inorganic Chemistry (5th ed.). Oxford: Clarendon Press.
- ↑ Brunvoll, J.; Ischenko, A. A.; Spiridonov, V. P.; Strand, T. G. (1984). "Composition and Molecular Structure of Gaseous Molybdenum Pentachloride by Electron Diffraction". Acta Chem. Scand. A38: 115–120. doi:10.3891/acta.chem.scand.38a-0115.
- ↑ Tamadon, Farhad; Seppelt, Konrad (2013). "The Elusive Halides VCl5, MoCl6, and ReCl6". Angew. Chem. Int. Ed. 52 (2): 767–769. doi:10.1002/anie.201207552. PMID 23172658.
- ↑ Dilworth, Jonathan R.; Richards, Raymond L. (1990). "The Synthesis of Molybdenum and Tungsten Dinitrogen Complexes". Inorganic Syntheses. Inorganic Syntheses. 28. pp. 33–43. doi:10.1002/9780470132593.ch7. ISBN 9780470132593.
- ↑ Calderazzo, Fausto; Maichle-Mössmer, Cäcilie; Pampaloni, Guido; Strähle, Joachim (1993). "Low-Temperature Syntheses of Vanadium(III) and Molybdenum(IV) Bromides by Halide Exchange". J. Chem. Soc., Dalton Trans. (5): 655–658. doi:10.1039/DT9930000655.
- ↑ Kauffmann, T.; Torii, S.; Inokuchi, T. (2004). "Molybdenum(V) Chloride". Encyclopedia of Reagents for Organic Synthesis. New York, NY: J. Wiley & Sons. doi:10.1002/047084289X. ISBN 9780471936237.
- ↑ Dilworth, Jonathan R.; Richards, Raymond L. (1990). The Synthesis of Molybdenum and Tungsten Dinitrogen Complexes. Inorganic Syntheses. 28. pp. 33–43. doi:10.1002/9780470132593.ch7. ISBN 9780470132593.
- ↑ Maria, Sébastien; Poli, Rinaldo (2014). "Ether Complexes of Molybdenum(III) and Molybdenum(IV) chlorides". Inorganic Syntheses: Volume 36. Inorganic Syntheses. 36. pp. 15–18. doi:10.1002/9781118744994.ch03. ISBN 9781118744994. https://hal.archives-ouvertes.fr/hal-02042499/file/332-10.1002-9781118744994.ch03-Accepted.pdf.
 |

