Chemistry:Molybdenum hexacarbonyl
|
| |||

| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Hexacarbonylmolybdenum(0)
| |||
| Systematic IUPAC name
Hexacarbonylmolybdenum[1] | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| EC Number |
| ||
| 3798, 562210 | |||
| MeSH | Hexacarbonylmolybdenum | ||
PubChem CID
|
|||
| UN number | 3466 | ||
| |||
| |||
| Properties | |||
| C6MoO6 | |||
| Molar mass | 264.01 g·mol−1 | ||
| Appearance | Vivid, white, translucent crystals | ||
| Density | 1.96 g cm−3 | ||
| Melting point | 150 °C (302 °F; 423 K) | ||
| Boiling point | 156 °C (313 °F; 429 K) | ||
| insoluble | |||
| Solubility | slightly soluble in THF, diglyme, acetonitrile[2] | ||
| Structure | |||
| Orthogonal | |||
| Octahedral | |||
| 0 D | |||
| Thermochemistry | |||
Std enthalpy of
formation (ΔfH⦵298) |
−989.1 kJ mol−1 | ||
Std enthalpy of
combustion (ΔcH⦵298) |
−2123.4 kJ mol−1 | ||
| Hazards | |||
| Safety data sheet | External MSDS | ||
| GHS pictograms | 
| ||
| GHS Signal word | Danger | ||
| H300, H310, H315, H319, H330, H413 | |||
| P261, P271, P280, P304+340+311Script error: No such module "Preview warning".Category:GHS errors, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Related compounds | |||
Related compounds
|
Chromium hexacarbonyl | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
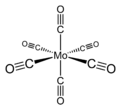
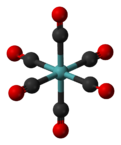
Molybdenum hexacarbonyl (also called molybdenum carbonyl) is the chemical compound with the formula Mo(CO)6. This colorless solid, like its chromium, tungsten, and seaborgium analogues, is noteworthy as a volatile, air-stable derivative of a metal in its zero oxidation state.
Structure and properties
Mo(CO)6 adopts an octahedral geometry consisting of six rod-like CO ligands radiating from the central Mo atom. A recurring minor debate in some chemical circles concerns the definition of an "organometallic" compound. Usually, organometallic indicates the presence of a metal directly bonded via a M–C bond to an organic fragment, which must in turn have a C–H bond.
Like many metal carbonyls, Mo(CO)6 is generally prepared by "reductive carbonylation", which involves reduction of a metal halide with under an atmosphere of carbon monoxide. As described in a 2023 survey of methods "most cost-effective routes for the synthesis of group 6 hexacarbonyls are based on the reduction of the metal chlorides (CrCl3, MoCl5 or WCl6) with magnesium, zinc or aluminium powders... under CO pressures".[4]
Occurrence
Mo(CO)6 has been detected in landfills and sewage plants, the reducing, anaerobic environment being conducive to formation of Mo(CO)6.[5]
Inorganic and organometallic research
Molybdenum hexacarbonyl is a popular reagent in academic research.[6]
One or more CO ligands can be displaced by other ligands.[7] Mo(CO)6, [Mo(CO)3(MeCN)3], and related derivatives are employed as catalysts in organic synthesis for example, alkyne metathesis and the Pauson–Khand reaction.
Mo(CO)6 reacts with 2,2′-bipyridine to afford Mo(CO)4(bipy). UV-photolysis of a THF solution of Mo(CO)6 gives Mo(CO)5(THF).
[Mo(CO)4(piperidine)2]
The thermal reaction of Mo(CO)6 with piperidine affords Mo(CO)4(piperidine)2. The two piperidine ligands in this yellow-colored compound are labile, which allows other ligands to be introduced under mild conditions. For instance, the reaction of [Mo(CO)4(piperidine)2] with triphenyl phosphine in boiling dichloromethane (b.p. ca. 40 °C) gives cis-[Mo(CO)4(PPh3)2]. This cis- complex isomerizes in toluene to trans-[Mo(CO)4(PPh3)2].[8]
[Mo(CO)3(MeCN)3]
Mo(CO)6 also can be converted to its tris(acetonitrile) derivative. The compound serves as a source of "Mo(CO)3". For instance treatment with allyl chloride gives [MoCl(allyl)(CO)2(MeCN)2], whereas treatment with KTp and sodium cyclopentadienide gives [MoTp(CO)3]− and [MoCp(CO)3]− anions, respectively. These anions react with a variety of electrophiles.[9] A related source of Mo(CO)3 is cycloheptatrienemolybdenum tricarbonyl.
Source of Mo atoms
Molybdenum hexacarbonyl is widely used in electron beam-induced deposition technique - it is easily vaporized and decomposed by the electron beam providing a convenient source of molybdenum atoms.[10]
Safety and handling
Like all metal carbonyls, Mo(CO)6 is a dangerous source of volatile metal as well as CO.
References
- ↑ "Hexacarbonylmolybdenum (CHEBI:30508)". Chemical Entities of Biological Interest (ChEBI). UK: European Bioinformatics Institute. https://www.ebi.ac.uk/chebi/searchId.do?chebiId=30508.
- ↑ Faller, John W.; Brummond, Kay M.; Mitasev, Branko (15 September 2006). "Hexacarbonylmolybdenum". Encyclopedia of Reagents for Organic Synthesis. Wiley. doi:10.1002/047084289X. ISBN 9780470842898. https://onlinelibrary.wiley.com/doi/10.1002/047084289X.rh004.pub2.
- ↑ Even, J.; Yakushev, A.; Dullmann, C. E.; Haba, H.; Asai, M.; Sato, T. K.; Brand, H.; Di Nitto, A. et al. (2014). "Synthesis and detection of a seaborgium carbonyl complex". Science 345 (6203): 1491–3. doi:10.1126/science.1255720. PMID 25237098. Bibcode: 2014Sci...345.1491E. (Subscription content?)
- ↑ Bruno, Sofia M.; Valente, Anabela A.; Gonçalves, Isabel S.; Pillinger, Martyn (2023). "Group 6 Carbonyl Complexes of N,O,P-Ligands as Precursors of High-Valent Metal-Oxo Catalysts for Olefin Epoxidation". Coordination Chemistry Reviews 478: 214983. doi:10.1016/j.ccr.2022.214983.
- ↑ Feldmann, J. (1999). "Determination of Ni(CO)4, Fe(CO)5, Mo(CO)6, and W(CO)6 in Sewage Gas by Using Cryotrapping Gas Chromatography Inductively Coupled Plasma Mass Spectrometry". Journal of Environmental Monitoring 1 (1): 33–37. doi:10.1039/a807277i. PMID 11529076.
- ↑ Faller, J. W.; Brummond, K. M.; Mitasev, B. (2006). Paquette, L.. ed. Encyclopedia of Reagents for Organic Synthesis. New York: J. Wiley & Sons. doi:10.1002/047084289X.rh004.pub2. ISBN 0471936235.
- ↑ "THE SYNTHESIS & SPECTROSCOPIC CHARACTERISATION OF METAL CARBONYL COMPLEXES". http://www.chm.bris.ac.uk/teaching-labs/inorganic2ndyear/2004-2005labmanual/Experiment3.pdf.
- ↑ Darensbourg, D. J.; Kump, R. L. (1978). "A Convenient Synthesis of cis-Mo(CO)4L2 Derivatives (L = Group 5a Ligand) and a Qualitative Study of Their Thermal Reactivity toward Ligand Dissociation". Inorg. Chem. 17 (9): 2680–2682. doi:10.1021/ic50187a062.
- ↑ Elschenbroich, C.; Salzer, A. (1992). Organometallics: A Concise Introduction (2nd ed.). Weinheim: Wiley-VCH. ISBN 3-527-28165-7.
- ↑ Randolph, S. J.; Fowlkes, J. D.; Rack, P. D. (2006). "Focused, Nanoscale Electron-Beam-Induced Deposition and Etching". Critical Reviews of Solid State and Materials Sciences 31 (3): 55–89. doi:10.1080/10408430600930438. Bibcode: 2006CRSSM..31...55R.
Further reading
- Marradi, M. (2005). "Synlett Spotlight 119: Molybdenum Hexacarbonyl [Mo(CO)6]". Synlett 2005 (7): 1195–1196. doi:10.1055/s-2005-865206. https://www.thieme-connect.de/ejournals/pdf/synlett/doi/10.1055/s-2005-865206.pdf.
- Feldmann, J.; Cullen, W. R. (1997). "Occurrence of Volatile Transition Metal Compounds in Landfill Gas: Synthesis of Molybdenum and Tungsten Carbonyls in the Environment". Environ. Sci. Technol. 31 (7): 2125–2129. doi:10.1021/es960952y. Bibcode: 1997EnST...31.2125F.
- Feldmann, J.; Grümping, R.; Hirner, A. V. (1994). "Determination of Volatile Metal and Metalloid Compounds in Gases from Domestic Waste Deposits with GC/ICP-MS". Fresenius' J. Anal. Chem. 350 (4–5): 228–234. doi:10.1007/BF00322474.
 |