Chemistry:Molybdenum hexafluoride
| |
| |
| Names | |
|---|---|
| IUPAC names
molybdenum(VI) fluoride
| |
| Other names
molybdenum hexafluoride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| MoF6 | |
| Molar mass | 209.93 g/mol |
| Appearance | white crystals[1] or colorless liquid hygroscopic |
| Density | 3.50 g/cm3[2] |
| Melting point | 17.5 °C (63.5 °F; 290.6 K)[1] |
| Boiling point | 34.0 °C (93.2 °F; 307.1 K)[1] |
| hydrolyzes | |
| −26.0·10−6 cm3/mol | |
| Structure | |
| Orthorhombic, oP28 | |
| Pnma, No. 62 | |
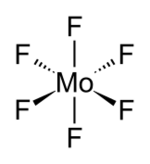
| octahedral (Oh) | |
| 0 | |
| Related compounds | |
Other cations
|
Tungsten hexafluoride Uranium hexafluoride Molybdenum(VI) chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Molybdenum hexafluoride, also molybdenum(VI) fluoride, is the inorganic compound with the formula MoF6. It is the highest fluoride of molybdenum. It is a colourless solid and melts just below room temperature and boils in 34 °C.[3] It is one of the seventeen known binary hexafluorides.
Synthesis
Molybdenum hexafluoride is made by direct reaction of molybdenum metal in an excess of elemental fluorine:[2]
- Mo + 3 F2 → MoF6
The compound hydrolyzes easily,[4] and typical impurities are MoO2F2 and MoOF4.[5]
Description
At −140 °C, it crystallizes in the orthorhombic space group Pnma. Lattice parameters are a = 9.394 Å, b = 8.543 Å, and c = 4.959 Å. There are four formula units (in this case, discrete molecules) per unit cell, giving a density of 3.50 g·cm−3.[2] The fluorine atoms are arranged in the hexagonal close packing.[6]
In liquid and gas phase, MoF6 adopt octahedral molecular geometry with point group Oh. The Mo–F bond length is 1.817 Å.[2]
Applications
Molybdenum hexafluoride has few uses. In the nuclear industry, MoF6 occurs as an impurity in uranium hexafluoride since molybdenum is a fission product of uranium.
The semiconductor industry constructs various integrated circuits through chemical vapor deposition of molybdenum hexafluoride.[4] In some cases, the deposited molybdenum is an impurity in the intended tungsten hexafluoride. MoF6 can be removed by reduction of a WF6-MoF6 mixture with any of a number of elements including hydrogen iodide at moderately elevated temperature.[7][8]
References
- ↑ 1.0 1.1 1.2 CRC Handbook of Chemistry and Physics, 90th Edition, CRC Press, Boca Raton, Florida, 2009, ISBN:978-1-4200-9084-0, Section 4, Physical Constants of Inorganic Compounds, p. 4-85.
- ↑ 2.0 2.1 2.2 2.3 T. Drews, J. Supeł, A. Hagenbach, K. Seppelt: "Solid State Molecular Structures of Transition Metal Hexafluorides", in: Inorganic Chemistry, 2006, 45 (9), S. 3782–3788; doi:10.1021/ic052029f; PMID 16634614
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ 4.0 4.1 Meshri., Dayal T. (2000), "Fluorine compounds, inorganic, molybdenum", Kirk-Othmer Encyclopedia of Chemical Technology, New York: John Wiley, doi:10.1002/0471238961.1315122513051908.a01, ISBN 9780471238966, http://onlinelibrary.wiley.com/book/10.1002/0471238961
- ↑ W. Kwasnik "Molybdenum(VI) Fluoride" Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 259.
- ↑ J. H. Levy, J. C Taylor, A. B. Waugh: "Neutron Powder Structural Studies of UF6, MoF6 and WF6 at 77 K", in: Journal of Fluorine Chemistry, 1983, 23 (1), pp. 29–36; doi:10.1016/S0022-1139(00)81276-2.
- ↑ US-Patent 5234679: Method of Refining Tungsten Hexafluoride Containing Molybdenum Hexafluoride as an Impurity , 10 August 1993
- ↑ US-Patent 6896866: Method for Purification of Tungsten Hexafluoride , 24 May 2005.
 |

