Chemistry:Olmesartan
 | |
| Clinical data | |
|---|---|
| Trade names | Benicar |
| Other names | Olmesartan medoxomil |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a603006 |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 26% |
| Metabolism | Liver (cannot be removed by hemodialysis) |
| Elimination half-life | 13 hours |
| Excretion | Kidney 40%, bile duct 60% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
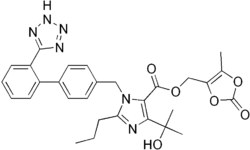
| Formula | C29H30N6O6 |
| Molar mass | 558.595 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Olmesartan, sold under the brand name Benicar among others, is a medication used to treat high blood pressure (hypertension).[1][2] It is taken by mouth.[2] Versions are available as the combination olmesartan/hydrochlorothiazide and olmesartan/amlodipine.[2]
Common side effects include dizziness, headaches, diarrhea, and back pain.[2] Serious side effects may include kidney problems, low blood pressure, and angioedema.[2] Use in pregnancy may harm the fetus and use when breastfeeding is not recommended.[3] It is an angiotensin II receptor antagonist and works by blocking the effects of angiotensin II.[2]
It was patented in 1991 and came into medical use in 2002.[4] It is available as a generic medication.[5] In 2020, it was the 139th most commonly prescribed medication in the United States, with more than 4 million prescriptions,[6] ranking 3rd among ARB's after Losartan (9th overall) and Valsartan (123rd overall).[7]
Medical uses
In the United States, olmesartan is indicated for the treatment of hypertension in people aged six years of age and older to lower blood pressure.[1]
Olmesartan is used for the treatment of hypertension. It may be used alone or in combination with other antihypertensive agents.[8] The US Food and Drug Administration (FDA) has determined that the benefits of olmesartan continue to outweigh its potential risks when used for the treatment of people with high blood pressure according to the drug label.[9]
Contraindications
Contraindications for treatment with olmesartan include biliary obstruction. Another major contraindication is pregnancy; reports in the scientific literature reveal fetal malformations for pregnant women taking sartan-derived drugs.[10]
Adverse effects
The incidence of adverse effects with olmesartan is reported as similar to placebo; the only adverse effect that occurred in >1% of patients treated with it and more frequently than placebo was dizziness (3% vs 1%). Rarely, olmesartan can cause severe gastrointestinal issues. The symptoms, which include nausea, vomiting, diarrhea, weight loss, and electrolyte abnormalities, are common among those who have celiac disease.[11] Recent studies suggested this form of sprue-like enteropathy could be caused by the inhibition of TGF-β, a polypeptide cytokine that maintains intestinal homeostasis. However, it is still unclear why this action was never observed with other ARBs.[12] In studies of angiotensin II receptor antagonists such as olmesartan, patients with unilateral or bilateral renal artery stenosis, increases in serum creatinine or blood urea nitrogen have been reported. There has been no long-term use of olmesartan medoxomil in patients with unilateral or bilateral renal artery stenosis, but similar results may be expected.[1]
Chemistry
An ester prodrug, it is completely and rapidly hydrolyzed to its active acid form.[13] The metabolites in this process are carbon dioxide and 2,3-dione.[citation needed]
History
It was patented in 1991 and came into medical use in 2002.[4]
Society and culture
Brand names
Olmesartan and Sevikar HCT combined is marketed worldwide by Daiichi Sankyo, in India by Abbott Healthcare Pvt. Ltd. under the trade name WinBP, by Zydus Cadila under the trade name Olmy, by Ranbaxy Laboratories Ltd. under the trade name Olvance, Olsar by Unichem Laboratories and in Canada by Schering-Plough as Olmetec. The marketing rights to the brand names Benicar, Benicar HCT, Azor, and Tribenzor in the United States were transferred from Daiichi Sankyo to Cosette in January 2022.[14]
Several preparations containing olmesartan and other antihypertensives are available. Teva Pharmaceuticals produces a formulation containing olmesartan, amlodipine, and hydrochlorothiazide.[15] Benicar HCT is the brand name of a medication containing olmesartan medoxomil with hydrochlorothiazide. Benitec H, another medication containing olmesartan medoxomil and hydrochlorothiazide, is marketed by GlaxoSmithKline in India.
Research
Olmesartan has demonstrated potential benefits in reducing the progression of atherosclerotic buildup in arteries. In large randomized placebo-controlled or active drug-controlled studies conducted in participants with hypertension, stable angina, or type 2 diabetes, long-term treatment with olmesartan has been shown to reduce the levels of markers of vascular inflammation.[16] This effect was also observed in a high-cholesterol primate test model.[17]
References
- ↑ Jump up to: 1.0 1.1 1.2 1.3 "Benicar- olmesartan medoxomil tablet, film coated". 7 September 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5b6f8a18-8f8a-4521-81c3-3a39c73c6646.
- ↑ Jump up to: 2.0 2.1 2.2 2.3 2.4 2.5 "Olmesartan Medoxomil Monograph for Professionals". American Society of Health-System Pharmacists. https://www.drugs.com/monograph/olmesartan-medoxomil.html.
- ↑ "Olmesartan Pregnancy and Breastfeeding Warnings". https://www.drugs.com/pregnancy/olmesartan.html.
- ↑ Jump up to: 4.0 4.1 Analogue-based Drug Discovery. John Wiley & Sons. 2006. p. 471. ISBN 9783527607495. https://books.google.com/books?id=FjKfqkaKkAAC&pg=PA471.
- ↑ British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 177. ISBN 9780857113382.
- ↑ "Olmesartan - Drug Usage Statistics". https://clincalc.com/DrugStats/Drugs/Olmesartan.
- ↑ "The Top 300 of 2020". https://clincalc.com/DrugStats/Top300Drugs.aspx.
- ↑ "Benicar (olmesartan medoxomil)". RxList Inc.. 5 July 2007. http://www.rxlist.com/benicar-drug.htm.
- ↑ "FDA Alert: Benicar (olmesartan): Ongoing Safety Review". Drugs.com. https://www.drugs.com/fda/benicar-olmesartan-ongoing-safety-review-12946.html.
- ↑ "Angiotensin II receptor blocker induced fetopathy: 7 cases". Klinische Padiatrie 223 (1): 10–14. January 2011. doi:10.1055/s-0030-1269895. PMID 21271514.
- ↑ "Histopathological changes in the gastrointestinal tract due to medications: an update for the surgical pathologist (part II of II)". International Journal of Surgical Pathology 22 (3): 202–211. May 2014. doi:10.1177/1066896913502230. PMID 24021900.
- ↑ "Severe spruelike enteropathy associated with olmesartan". Mayo Clinic Proceedings 87 (8): 732–738. August 2012. doi:10.1016/j.mayocp.2012.06.003. PMID 22728033.
- ↑ "An update on non-peptide angiotensin receptor antagonists and related RAAS modulators". Life Sciences 81 (8): 615–639. August 2007. doi:10.1016/j.lfs.2007.06.007. PMID 17692338.
- ↑ "Cosette Pharmaceuticals Acquires Rights to Eight Branded Products from Daiichi Sankyo". Cosette Pharma (Press release). 18 January 2022. Retrieved 14 April 2023.
- ↑ "OLMESARTAN MEDOXOMIL, AMLODIPINE AND HYDROCHLOROTHIAZIDE – olmesartan medoxomil, amlodipine and hydrochlorothiazide tablet, film coated". U.S. National Library of Medicine. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5c128ef2-60a6-40f3-b37f-ee139fe27987.
- ↑ "Management of arterial hypertension with angiotensin receptor blockers: Current evidence and the role of olmesartan". Cardiovascular Therapeutics 36 (6): e12471. December 2018. doi:10.1111/1755-5922.12471. PMID 30358114.
- ↑ "Anti-atherosclerotic efficacy of olmesartan". Journal of Human Hypertension 16 (Suppl 2): S7-12. May 2002. doi:10.1038/sj.jhh.1001393. PMID 11967727.
 |

