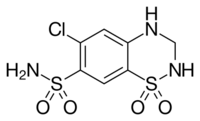
Chemistry:Hydrochlorothiazide
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Hydrodiuril, others |
| Other names | HCTZ, HCT |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682571 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Variable (~70% on average) |
| Metabolism | Not significant[2] |
| Elimination half-life | 5.6–14.8 h |
| Excretion | Primarily kidney (>95% as unchanged drug) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C7H8ClN3O4S2 |
| Molar mass | 297.73 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Hydrochlorothiazide, sold under the brand name Hydrodiuril among others, is a diuretic medication used to treat hypertension and swelling due to fluid build-up.[3] Other uses include treating diabetes insipidus and renal tubular acidosis and to decrease the risk of kidney stones in those with a high calcium level in the urine.[3] Hydrochlorothiazide is taken by mouth and may be combined with other blood pressure medications as a single pill to increase effectiveness.[3] Hydrochlorothiazide is a thiazide medication which inhibits reabsorption of sodium and chloride ions from the distal convoluted tubules of the kidneys, causing a natriuresis.[3][4] This initially increases urine volume and lowers blood volume.[5] It is believed to reduce peripheral vascular resistance.[5]
Potential side effects include poor kidney function, electrolyte imbalances, including low blood potassium, and, less commonly, low blood sodium, gout, high blood sugar, and feeling lightheaded with standing.[3]
Two companies, Merck & Co. and Ciba Specialty Chemicals, state they discovered the medication which became commercially available in 1959.[6] It is on the World Health Organization's List of Essential Medicines.[7] It is available as a generic drug[3] and is relatively affordable.[8] In 2021, it was the twelfth most commonly prescribed medication in the United States, with more than 39 million prescriptions.[9][10]
Medical uses
Hydrochlorothiazide is used for the treatment of hypertension, congestive heart failure, symptomatic edema, diabetes insipidus, renal tubular acidosis.[3] It is also used for the prevention of kidney stones in those who have high levels of calcium in their urine.[3]
Multiple studies suggest hydrochlorothiazide could be used as initial monotherapy in people with primary hypertension; however, the decision should be weighed against the consequence of long-term adverse metabolic abnormalities.[11][12] Doses of hydrochlorothiazide of 50 mg or less over four years reduced mortality and development of cardiovascular diseases better than high-dose hydrochlorothiazide (50 mg or more) and beta-blockers.[4] A 2019 review supported equivalence between drug classes for initiating monotherapy in hypertension, although thiazide or thiazide-like diuretics showed better primary effectiveness and safety profiles than angiotensin-converting enzyme inhibitors and non-dihydropyridine calcium channel blockers.[11]
Low doses (50 mg or less) of hydrochlorothiazide as first‐line therapy for hypertension were found to reduce total mortality and cardiovascular disease events over a four-year study.[4] Hydrochlorothiazide appears be more effective than chlorthalidone in preventing heart attacks and strokes.[13] Hydrochlorothiazide is less potent but may be more effective than chlorthalidone in reducing blood pressure.[13][14] More robust studies are required to confirm which drug is superior in reducing cardiovascular events.[15] Side effect profile for both drugs appear similar and are dose dependent.[13]
Hydrochlorothiazide is also sometimes used to prevent osteopenia and treat hypoparathyroidism,[16] hypercalciuria, Dent's disease, and Ménière's disease.
A low level of evidence, predominantly from observational studies, suggests that thiazide diuretics have a modest beneficial effect on bone mineral density and are associated with a decreased fracture risk when compared with people not taking thiazides.[17][18][19] Thiazides decrease mineral bone loss by promoting calcium retention in the kidney, and by directly stimulating osteoblast differentiation and bone mineral formation.[20]
The combination of fixed-dose preparation such as losartan/hydrochlorothiazide has added advantages of a more potent antihypertensive effect with additional antihypertensive efficacy at the dose of 100 mg/25 mg when compared to monotherapy.[21][22]
Adverse effects
- Hypokalemia, or low blood levels of potassium are an occasional side effect. It can be usually prevented by potassium supplements or by combining hydrochlorothiazide with a potassium-sparing diuretic
- Other disturbances in the levels of serum electrolytes, including hypomagnesemia (low magnesium), hyponatremia (low sodium), and hypercalcemia (high calcium)
- Hyperuricemia (high levels of uric acid in the blood). All thiazide diuretics including hydrochlorothiazide can inhibit excretion of uric acid by the kidneys, thereby increasing serum concentrations of uric acid. This may increase the incidence of gout in doses of ≥ 25 mg per day and in more susceptible patients such as male gender of <60 years old.[22][23][24]
- Hyperglycemia, high blood sugar
- Hyperlipidemia, high cholesterol and triglycerides
- Headache
- Nausea/vomiting
- Photosensitivity
- Weight gain
- Pancreatitis
Package inserts contain vague and inconsistent data surrounding the use of thiazide diuretics in patients with allergies to sulfa drugs, with little evidence to support these statements.[25] A retrospective cohort study conducted by Strom et al. concluded that there is an increased risk of an allergic reaction occurring in patients with a predisposition to allergic reactions in general rather than cross reactivity from structural components of the sulfonamide-based drug.[26] Prescribers should examine the evidence carefully and assess each patient individually, paying particular attention to their prior history of sulfonamide hypersensitivity rather than relying on drug monograph information.[27]
There is an increased risk of non-melanoma skin cancer.[28] In August 2020, the Australian Therapeutic Goods Administration required the Product Information (PI) and Consumer Medicine Information (CMI) for medicines containing hydrochlorothiazide to be updated to include details about an increased risk of non-melanoma skin cancer.[29] In August 2020, the U.S. Food and Drug Administration (FDA) updated the drug label about an increased risk of non-melanoma skin cancer (basal cell skin cancer or squamous cell skin cancer).[30]
Society and culture


Brand names
Hydrochlorothiazide is available as a generic drug under a large number of brand names, including Apo-Hydro, Aquazide, BPZide, Dichlotride, Esidrex, Hydrochlorot, Hydrodiuril, HydroSaluric, Hypothiazid, Microzide, Oretic and many others.
To reduce pill burden and in order to reduce side effects, hydrochlorothiazide is often used in fixed-dose combinations with many other classes of antihypertensive drugs such as:
- ACE inhibitors — e.g. Prinzide or Zestoretic (with lisinopril), Co-Renitec (with enalapril), Capozide (with captopril), Accuretic (with quinapril), Monopril HCT (with fosinopril), Lotensin HCT (with benazepril), etc.
- Angiotensin receptor blockers — e.g. Hyzaar (with losartan), Co-Diovan or Diovan HCT (with valsartan), Teveten Plus (with eprosartan), Avalide or CoAprovel (with irbesartan), Atacand HCT or Atacand Plus (with candesartan), etc.
- Beta blockers — e.g. Ziac or Lodoz (with bisoprolol),[31] Nebilet Plus or Nebilet HCT (with nebivolol), Dutoprol or Lopressor HCT (with metoprolol), etc.
- Direct renin inhibitors — e.g. Co-Rasilez or Tekturna HCT (with aliskiren)
- Potassium sparing diuretics: Dyazide and Maxzide triamterene[32]
Sport
Use of hydrochlorothiazide is prohibited by the World Anti-Doping Agency for its ability to mask the use of performance-enhancing drugs.[33]
References
- ↑ "Hydrochlorothiazide Use During Pregnancy". 30 July 2019. https://www.drugs.com/pregnancy/hydrochlorothiazide.html.
- ↑ "Absorption, metabolism, and excretion of hydrochlorothiazide". Clinical Pharmacology and Therapeutics 19 (5 Pt 1): 531–537. May 1976. doi:10.1002/cpt1976195part1531. PMID 1277708.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 "Hydrochlorothiazide". Drugs.com. 15 November 2022. https://www.drugs.com/monograph/hydrochlorothiazide.html.
- ↑ 4.0 4.1 4.2 "First-line drugs for hypertension". The Cochrane Database of Systematic Reviews 2018 (4): CD001841. April 2018. doi:10.1002/14651858.CD001841.pub3. PMID 29667175.
- ↑ 5.0 5.1 "Mechanisms for blood pressure lowering and metabolic effects of thiazide and thiazide-like diuretics". Expert Review of Cardiovascular Therapy 8 (6): 793–802. June 2010. doi:10.1586/erc.10.27. NIHMSID: NIHMS215063. PMID 20528637.
- ↑ The evolution of drug discovery: from traditional medicines to modern drugs (1st ed.). Weinheim: Wiley-VCH. 2011. p. 74. ISBN 9783527326693. https://books.google.com/books?id=iDNy0XxGqT8C&pg=PA74.
- ↑ World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. 2021. WHO/MHP/HPS/EML/2021.02.
- ↑ "Best drugs to treat high blood pressure The least expensive medications may be the best for many people". November 2014. http://www.consumerreports.org/cro/2011/03/best-drugs-to-treat-high-blood-pressure/index.htm.
- ↑ "The Top 300 of 2021". https://clincalc.com/DrugStats/Top300Drugs.aspx.
- ↑ "Hydrochlorothiazide - Drug Usage Statistics". https://clincalc.com/DrugStats/Drugs/Hydrochlorothiazide.
- ↑ 11.0 11.1 "Comprehensive comparative effectiveness and safety of first-line antihypertensive drug classes: a systematic, multinational, large-scale analysis". Lancet 394 (10211): 1816–1826. November 2019. doi:10.1016/S0140-6736(19)32317-7. PMID 31668726.
- ↑ "Pharmacotherapy for hypertension in adults aged 18 to 59 years". The Cochrane Database of Systematic Reviews 2017 (8): CD008276. August 2017. doi:10.1002/14651858.CD008276.pub2. PMID 28813123.
- ↑ 13.0 13.1 13.2 "Comparison of Cardiovascular and Safety Outcomes of Chlorthalidone vs Hydrochlorothiazide to Treat Hypertension". JAMA Internal Medicine 180 (4): 542–551. April 2020. doi:10.1001/jamainternmed.2019.7454. PMID 32065600.
- ↑ "Meta-analysis of dose-response relationships for hydrochlorothiazide, chlorthalidone, and bendroflumethiazide on blood pressure, serum potassium, and urate". Hypertension 59 (6): 1104–1109. June 2012. doi:10.1161/HYPERTENSIONAHA.111.190637. PMID 22547443.
- ↑ "Chlorthalidone reduces cardiovascular events compared with hydrochlorothiazide: a retrospective cohort analysis". Hypertension 57 (4): 689–694. April 2011. doi:10.1161/HYPERTENSIONAHA.110.161505. PMID 21383313.
- ↑ "Long-term follow-up of patients with hypoparathyroidism". The Journal of Clinical Endocrinology and Metabolism 97 (12): 4507–4514. December 2012. doi:10.1210/jc.2012-1808. PMID 23043192.
- ↑ "Thiazide diuretics and the risk of hip fracture". The Cochrane Database of Systematic Reviews (10): CD005185. October 2011. doi:10.1002/14651858.CD005185.pub2. PMID 21975748.
- ↑ "Thiazide diuretic usage and risk of fracture: a meta-analysis of cohort studies". Osteoporosis International 29 (7): 1515–1524. July 2018. doi:10.1007/s00198-018-4486-9. PMID 29574519.
- ↑ "Bone mineral density changes among women initiating blood pressure lowering drugs: a SWAN cohort study". Osteoporosis International 27 (3): 1181–1189. March 2016. doi:10.1007/s00198-015-3332-6. PMID 26449354.
- ↑ "Thiazide diuretics directly induce osteoblast differentiation and mineralized nodule formation by interacting with a sodium chloride co-transporter in bone". Journal of the American Society of Nephrology 18 (9): 2509–2516. September 2007. doi:10.1681/ASN.2007030348. PMID 17656470.
- ↑ "Antihypertensive effects of two fixed-dose combinations of losartan and hydrochlorothiazide versus hydrochlorothiazide monotherapy in subjects with ambulatory systolic hypertension". American Journal of Hypertension 16 (12): 1036–1042. December 2003. doi:10.1016/j.amjhyper.2003.07.014. PMID 14643578.
- ↑ 22.0 22.1 "Blood pressure-lowering efficacy of monotherapy with thiazide diuretics for primary hypertension". The Cochrane Database of Systematic Reviews 2014 (5): CD003824. May 2014. doi:10.1002/14651858.cd003824.pub2. PMID 24869750.
- ↑ "Use of diuretics and the risk of gouty arthritis: a systematic review". Seminars in Arthritis and Rheumatism 41 (6): 879–889. June 2012. doi:10.1016/j.semarthrit.2011.11.008. PMID 22221907.
- ↑ "Comparison of new-onset gout in adults prescribed chlorthalidone vs. hydrochlorothiazide for hypertension". Journal of Clinical Hypertension 16 (12): 864–868. December 2014. doi:10.1111/jch.12413. PMID 25258088.
- ↑ "Sulfonamide cross-reactivity: fact or fiction?". The Annals of Pharmacotherapy 39 (2): 290–301. February 2005. doi:10.1345/aph.1E350. PMID 15644481.
- ↑ "Absence of cross-reactivity between sulfonamide antibiotics and sulfonamide nonantibiotics". The New England Journal of Medicine 349 (17): 1628–1635. October 2003. doi:10.1056/NEJMoa022963. PMID 14573734.
- ↑ "An evidence-based approach for providing cautionary recommendations to sulfonamide-allergic patients and determining cross-reactivity among sulfonamide-containing medications". Journal of Clinical Pharmacy and Therapeutics 38 (3): 196–202. June 2013. doi:10.1111/jcpt.12048. PMID 23489131.
- ↑ "Hydrochlorothiazide use and risk of nonmelanoma skin cancer: A nationwide case-control study from Denmark". Journal of the American Academy of Dermatology 78 (4): 673–681.e9. April 2018. doi:10.1016/j.jaad.2017.11.042. PMID 29217346.
- ↑ "Hydrochlorothiazide". 24 August 2020. https://www.tga.gov.au/alert/hydrochlorothiazide.
- ↑ "FDA approves label changes to hydrochlorothiazide". 20 August 2020. https://www.fda.gov/drugs/drug-safety-and-availability/fda-approves-label-changes-hydrochlorothiazide-describe-small-risk-non-melanoma-skin-cancer.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "List of nationally authorised medicinal products : Active substance: bisoprolol / hydrochlorothiazide Procedure no.: PSUSA/00000420/202111". https://www.ema.europa.eu/documents/psusa/bisoprolol/hydrochlorothiazide-list-nationally-authorised-medicinal-products-psusa/00000420/202111_en.pdf.
- ↑ "Triamterene and Hydrochlorothiazide". 1 January 2020. https://medlineplus.gov/druginfo/meds/a601125.html.
- ↑ "Prohibited List". World Anti-Doping Agency. January 2018. https://www.wada-ama.org/sites/default/files/prohibited_list_2018_en.pdf.
 |
